1. INTRODUCTION
2. MATERIALS AND METHODS
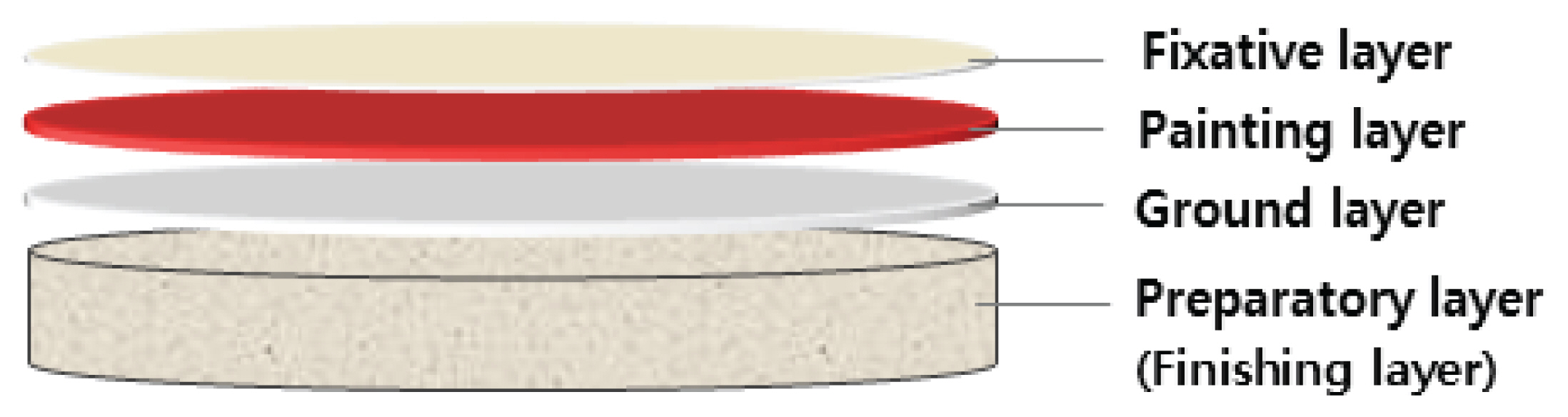
2.1. Mock-up samples of mural painting
2.2. Gel cleaners
2.3. Evaluation methods
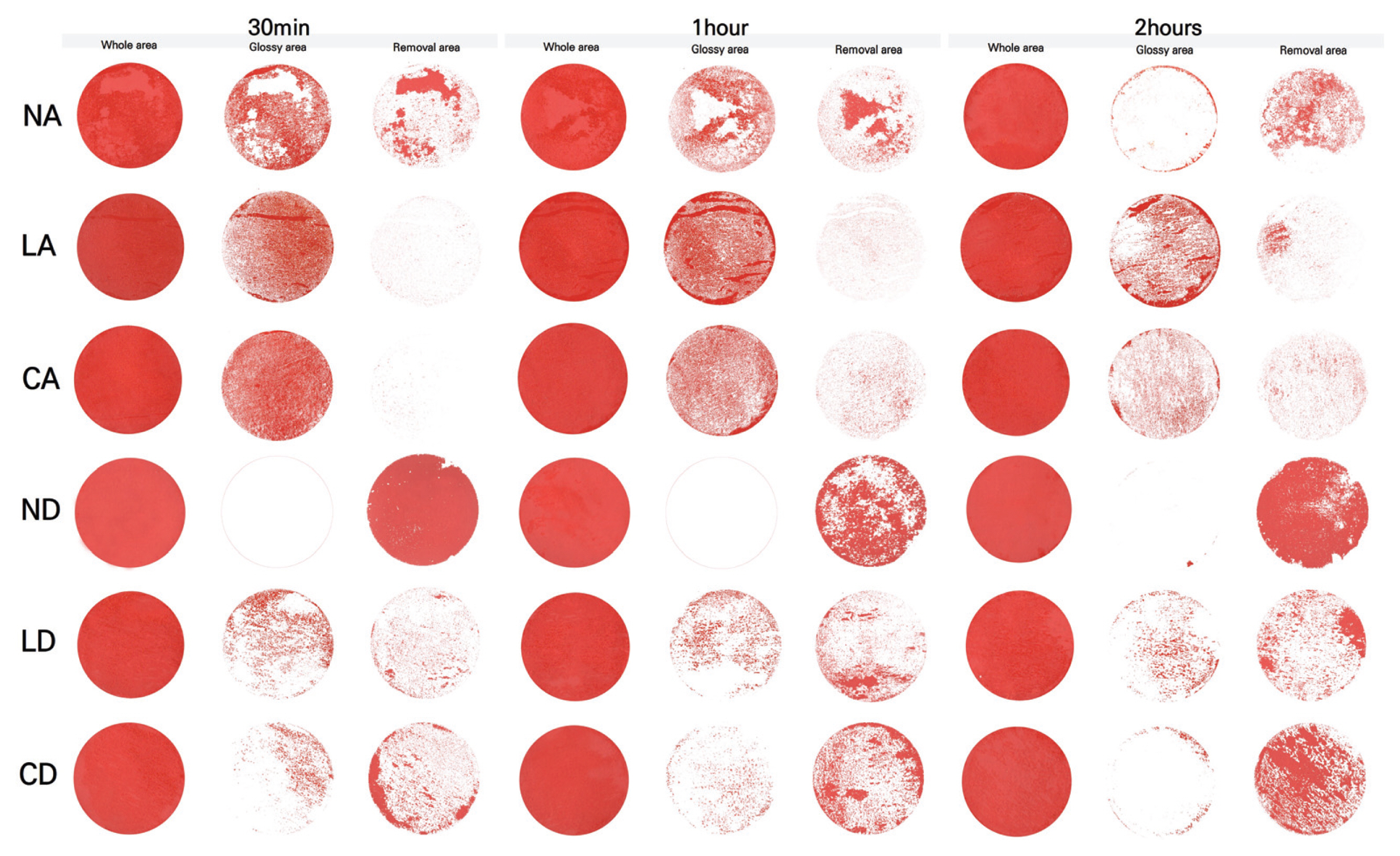
2.3.1. Surface observation
2.3.2. Chromaticity
2.3.3. Glossiness
3. RESULT AND DISCUSSION
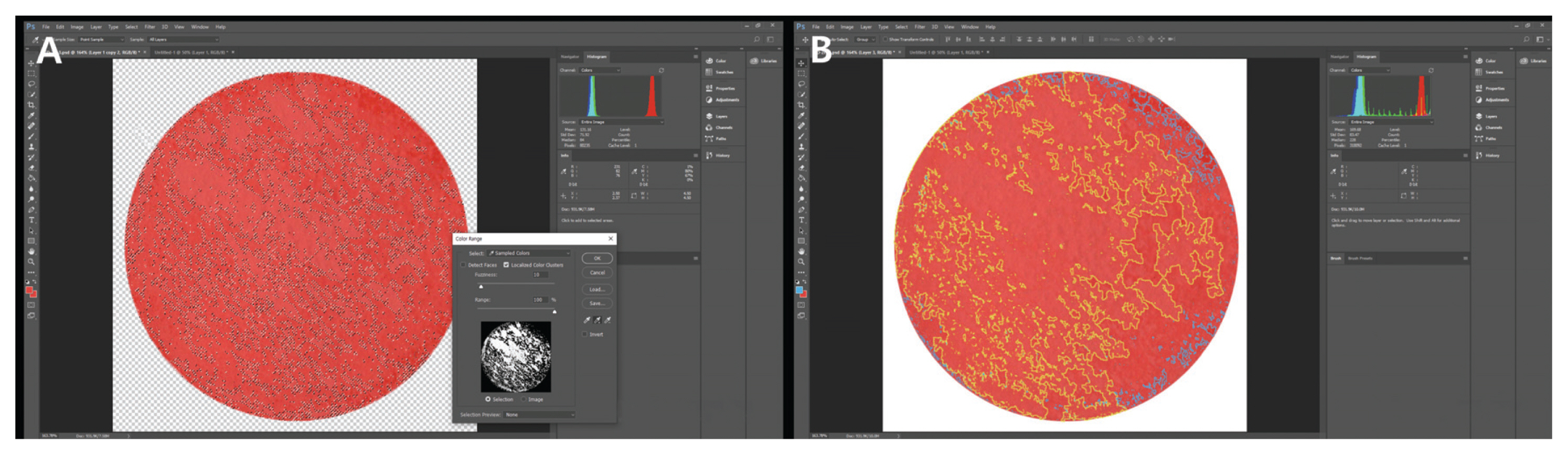
3.1. Microscopy
3.2. Chromaticity
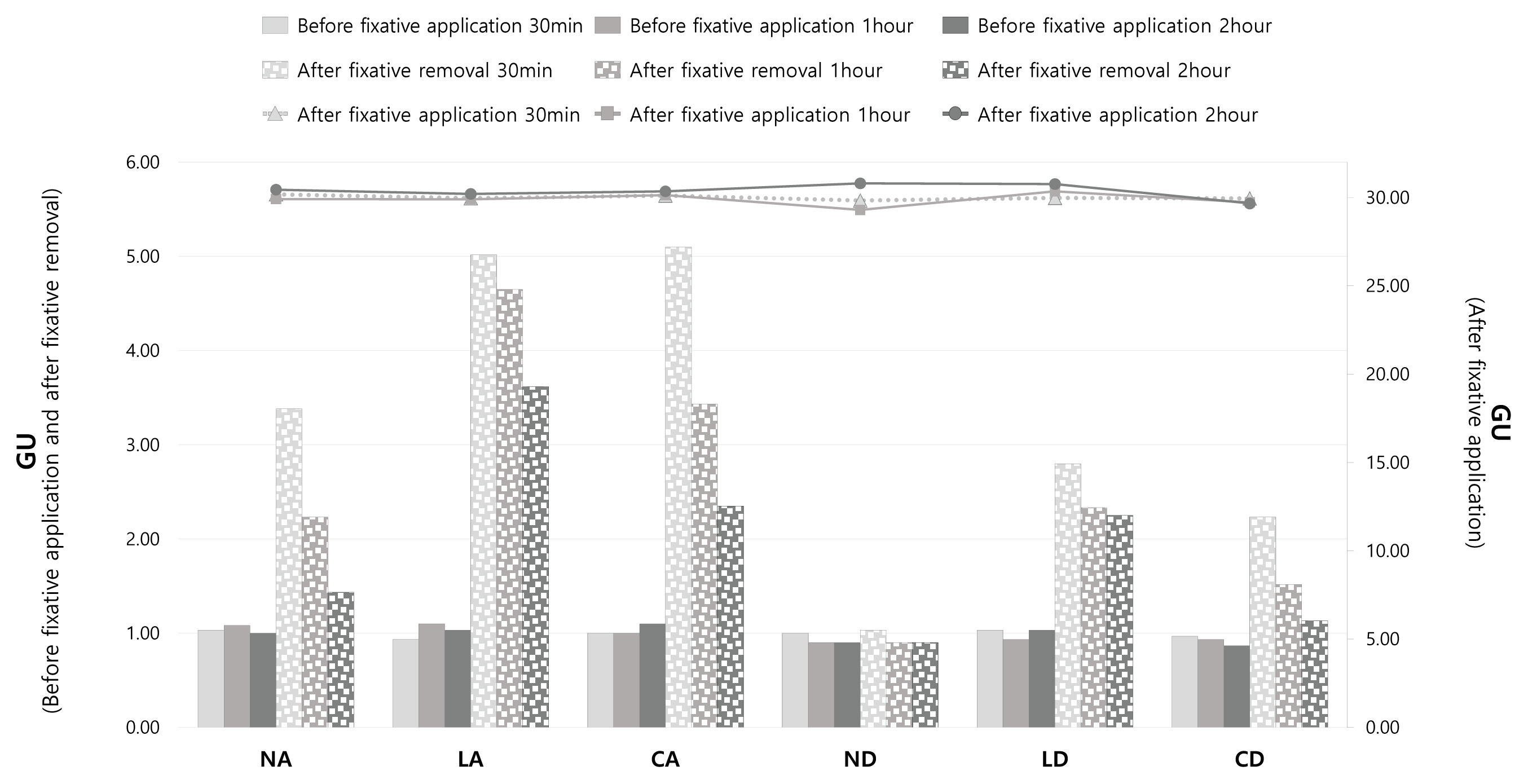
3.3. Glossiness
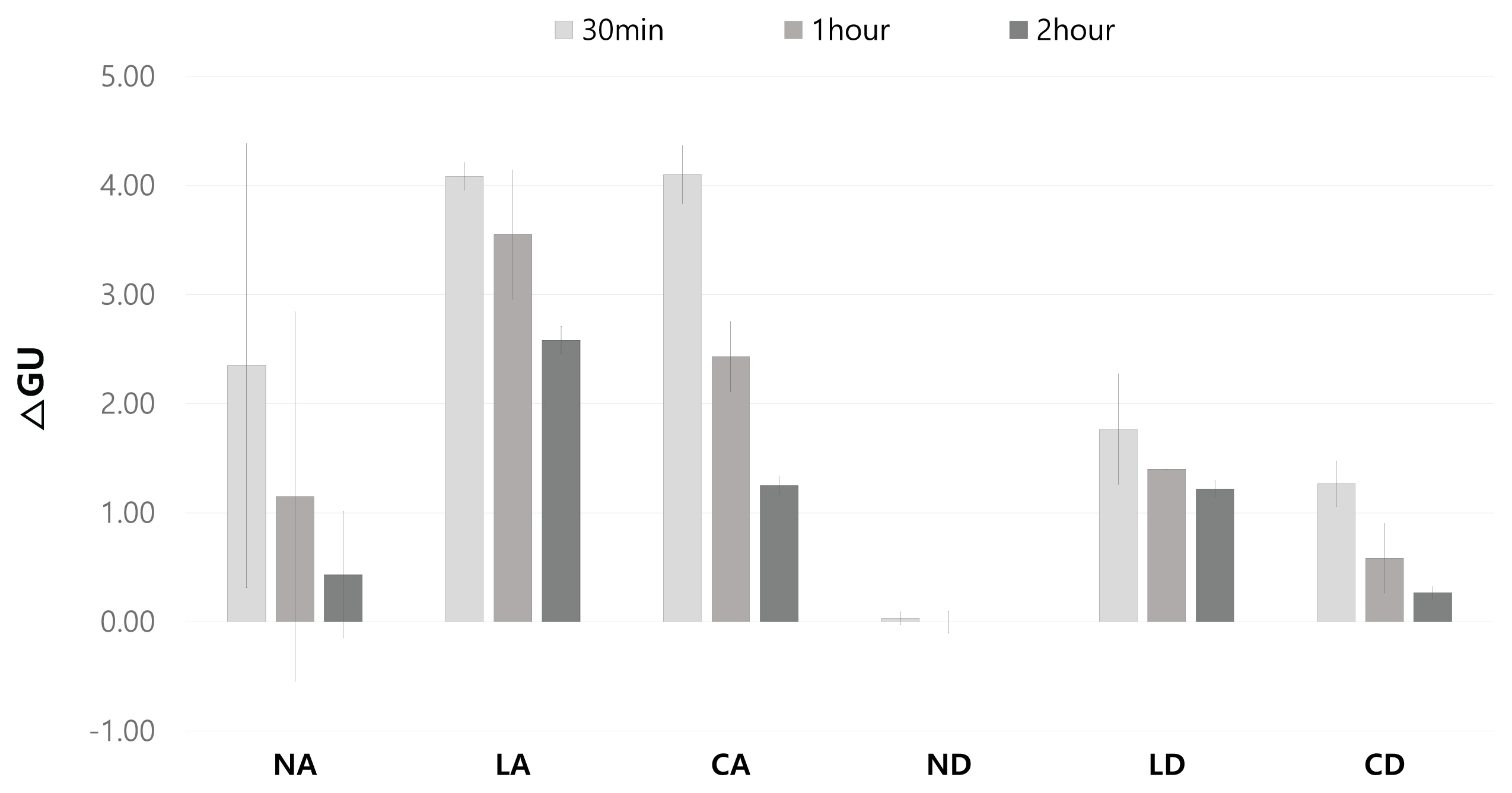
3.4. Gloss and removal area
4. CONCLUSION
In previous Part 1 of the study (Yu et al., 2020), the results of the evaluation of the removal power of the gel cleaners showed that the removal ratio of the cleaner mixed with dimethyl carbonate was higher than that mixed with acetone, and the removal effect of the gelling agents was in the order of Nevek® > Carbopol®980 > Laponite®RD when the same solvent was used. The removal ratio increased as the application time of the gel cleaner increased.
After comprehensively investigating the removal effect in terms of color changes, glossiness changes, and removal status for the applicability of the gel cleaners to the mock-up samples in the conditions similar to those of the mural painting of the Payathonzu temple, this study found that the gel cleaner mixed with dimethyl carbonate had a superior removal effect to the gel cleaner mixed with acetone. Furthermore, for the same solvent, the gelling agent showed the removal effect in the order of Nevek® > Carbopol®980 > Laponite®RD. In the condition of mixing Carbopol®980 and dimethyl carbonate and the condition of mixing Laponite®RD and dimethyl carbonate, the removal effect increased as the application time increased, but in the condition of mixing Nevek® and dimethyl carbonate, the removal effect was achieved in 30 minutes was the highest.
This study found that the cleaning effect of a gel cleaner was influenced by the solubility and volatility of the solvent to the fixative and the solvent release properties of the gelling agent. Even if a gel cleaner had a superior removal power, its removal effect may vary depending on the application target and time.
The gel cleaning system studied in this experiment has a removal effect on poly(vinyl acetate) fixative, and enabled selective cleaning by selectively applying the conditions that might control the cleaning action while reducing excessive solvent emissions.