Off-gassing Woodblock Prints - Storage Impact Considerations and Mitigation Strategies -
Article information
Abstract
The storage environment of a Japanese woodblock print collection was assessed for organic acids. The active air sampling method was used to collect organic acid emissions in the low microgram range from areas of a selected woodblock print with different pigments, following which an off-gassing mitigation strategy based on the fan filter unit(FFU) system was investigated. Research findings revealed that the off-gassing behavior of woodblock prints is significantly impacted by storage practices and to a lesser degree by the pigments. The FFU system can be used as a mitigation strategy, but the permanence of the results depends on the storage conditions.
1. INTRODUCTION
Organic acids—particularly acetic acid and formic acid— are known to be released as degradation products from paper and wood materials, and they are often found in comparatively higher quantities inside libraries(Gibson et al., 2012; Mašková et al., 2017). When no mitigation strategy is employed, the released gases may accumulate, especially in enclosed spaces, and eventually attack collection materials (Tétreault et al., 1998; Tétreault et al., 2003;Brokerhof and Van Bommel, 1996;Gibson and Watt, 2010;Oikawa et al., 2006;Malagodi et al., 2017). To minimize potential damage, many museum institutions have issued guidelines to monitor organic acids and other volatile organic compound(VOC) emissions. The Tokyo National Museum(TNM), for instance, recommends the maintenance of acetic acid and formic acid concentrations below 123 µg/m³ and 19 µg/m³ indoors, respectively.
Being common gases released from paper materials, organic acids tend to accumulate inside paper-made storage boxes, especially if the boxes are used to store paper-based collections. As such, woodblock prints, which are typically stored in wooden or cardboard boxes and sandwiched in Japanese traditional paper folders, are likely to be exposed to organic acid-concentrated microclimates. It is therefore important to evaluate the storage conditions in terms of VOCs and develop methods for mitigating off-gassing.
In this paper, we present the results of an investigation on the storage conditions of a Meiji period(1868–1912) woodblock print collection in terms of the concentration of gaseous organic acids. We also assess the print’s off-gassing behavior in different colored areas, which were characterized by an X-ray fluorescence(XRF) analyzer. In addition, we describe an organic acid rinse experiment performed on a selected woodblock print using the fan filter unit(FFU) system. The FFU system had been used in a previous experiment to successfully reduce organic acid off-gassing in Japanese folding screens by 85%(Matsui et al., 2018). In this experiment, the results obtained from gas rinsing were evaluated after a 20-25 day follow-up period.
2. MATERIALS: WOODBLOCK PRINTS
The woodblock prints studied in this research belong to a three-print set titled “Yōjo Reishiki Kyōiku-no-zu”, which was attributed to Toyohara Chikanobu(1838–1912). These prints(dim.: 37 × 24 cm) were acquired by the University of Tsukuba Library in 2009 and, since acquisition, have been preserved inside a hōsho paper folder(high-quality Japanese traditional paper fabricated from mulberry bast fibers), where they are piled in traditional order(the right-side print is at the top of the stack) and stored inside a wooden box 47 cm × 36 cm × 11 cm in size, with an approximate volume capacity of 13,770 cm3. It was not possible to obtain information on the handling history of the prints prior to their acquisition by the library. However, it is known that since their acquisition, they have, for the most part, been stored inside a wooden box, having been retrieved only once for a temporary one-month exhibition. Aside from this group of prints, 19 other hōsho paper folders, each housing other sets of woodblock prints attributed to different artists of the Meiji period, are also stored in the box.
3. METHOD
3.1. Determination of sampling points and characterization of color materials
The middle and right-side prints of the three-print set were measured for organic acid emissions. Two sampling points(M-03, M-04) in the middle print, representing two different colored areas(red and blue), and four sampling points(M-01, M-02, M-05, M-06) in the right-side print, representing four different colored areas(yellow, black, green and red), were selected for the off-gassing measurements. The location of the sampling points is shown in Figure 1.
The color materials of the selected sampling points were characterized using a Brucker Tracer III-SD handheld X-ray fluorescence(XRF)(Tracer III-SD, Brucker, USA) analyzer equipped with a rhodium target X-ray tube set at 40 kV and 11 µA. Each sample was scanned for 60 seconds. XRF scanning was conducted twice for each location to verify the consistency of the results. It was not always possible to scan the exact area of the sampling point owing to it being too small or having traces of other nearby colors that could interfere with the results; therefore, nearby areas that displayed the same color were analyzed instead. Apart from characterization of the color materials in the sampling points, we also conducted XRF analysis in other colored areas of the prints for reference. The location of the scanned areas is shown in Figure 1.
During scanning, to protect the pigments of the fragile prints, a cushion fabricated of two sheets of Kim Towel and one Art-Sorb® sheet was set up, and the print was placed face down on top of it. XRF analysis was conducted through the rear side of the print. The cushion surface, as well as an area of the paper with no color materials, were also analyzed with XRF for background characterization.
Finally, the selected colored areas used as sampling points and/or scanned by the XRF analyzer were also measured using a Konica Minolta’s portable spectrophotometer (CM-2600d, Konica Minolta, JPN) with the specular component excluded(SCE), a 3-mm dimeter aperture, a standard illuminant D65(6500 K), 0% UV, and a 10° observer angle to obtain their CIE L*, a*, and b* values.
3.2. Measuring organic acids
Measurement of organic acid content(acetic acid and formic acid) was performed via active air sampling using Kitagawa™ precision gas no. 910 detector tubes. These detector tubes exhibit a detection range of 5 µg/m³ and 400 µg/m³ for acetic acid, and 5 µg/m³ and 800 µg/m³ for formic acid, and are equipped with a measuring scale printed alongside the tubes that is calibrated to acetic acid concentrations at 20°C. Measurements recorded under different ambient air temperature conditions required the application of temperature corrections to the obtained readings, based on the temperature reference chart provided by the manufacturer. To perform the measurements, the detector tubes were mounted on an ASP-1200 air sampling pump. As the tubes were designed to collect air at a flow rate of 200 ml/min for 60 min, the pump settings were adjusted to match the tubes’ specifications, and a total amount of 12 L of air was sampled for each measurement.
Off-gassing measurement in the prints was conducted at the sampling points(M-01 to M-06) as previously described. To sample the print, an apparatus composed of two acrylic sheets(3 mm thickness), with 3.5 mm diameter holes for sampling and a metal rack support, was constructed as illustrated in Figure 2. This allowed for a measuring surface area of 7 mm2. The print was adjusted between the acrylic sheets so that the area to be sampled interfaced with the sampling hole, where the tip of the detector tube was inserted. The measurement was performed through the rear side of the print.
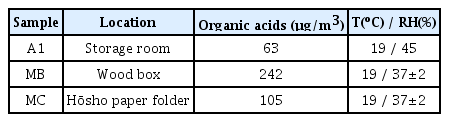
In addition to measuring off-gassing from the prints, the wooden box(in closed state) and the hōsho paper folder were sampled for volatile organic acid content, along with the air in the storage room(Figure 3). Measurements in the wooden box and paper folder were not conducted in situ; instead, the materials were taken to another room with a low organic acid concentration for sample collection(blank = 20±3 µg/m3).
3.3. Rinsing organic acids: experimental design
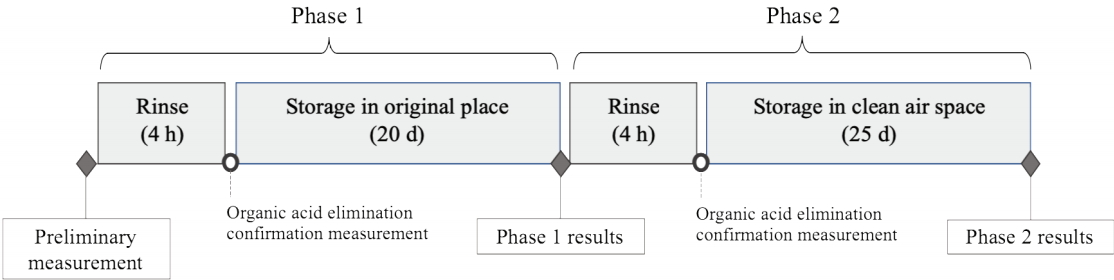
The following experiment plan was designed to evaluate the influence of storage environment on organic acid off-gassing and assess the short-time impact of using the FFU system as a mitigation strategy. Data collection was conducted in three steps: during preliminary measurement (before rinse experiment), after Phase 1 of the experiment, and after Phase 2 of the experiment. For this experiment, the right-side woodblock print was used, and its four sampling points(M-01, M-02, M-05, M-06) were measured for changes in organic acid content before the experiment, after Phase 1, and after Phase 2.
The rinse experiment involved two Phases. In Phase 1, the organic acid content was rinsed off of the woodblock print, following which the print was returned to its original storage place, where it remained for 20 d, after which organic acid re-emission was measured. In Phase 2, executed after Phase 1 was concluded, the woodblock print was rinsed again; this time, it was subsequently stored in a different storage place, i.e., inside a closed polypropylene container with an Art-Sorb® silica cassette for humidity control, in a room with low organic acid content in air(<22 µg/m³) for 25 d. Figure 4 shows the procedure followed in the experiment.
The organic acid rinse procedure was conducted via an FFU system. A Nippon Muki fan-filter unit, PFT2 model (dim: 250 mm × 250 mm × 65 mm), equipped with three chemical filters for alkali gases, acidic gases, and organic gases was used to create a closed, recirculating stream of clean air to rinse the woodblock print and eliminate its organic acid emissions(Figure 5). The FFU produced an average airflow of 68 m3/h. The print(supported by a metal stand), the FFU, and the two Art-Sorb® silica cassettes for humidity control were sealed inside a bag made of an Escal® gas-barrier film(ceramic deposited super barrier film developed by Mitsubishi Gas Chemical Co.) with plastic clips. The volume inside the bag was approximately 93 L. A metal rack(dim: 29 cm × 31 cm × 79 cm) was also placed inside to prevent the gas-barrier film from clinging to the print and to permit better airflow(Figure 6). The rinsing procedure was concluded in 4 h. The total volume of air utilized in this experiment is estimated to be 272 kL, with 720 air changes per hour. After the rinse program was concluded, the print was measured for off-gassing at two sampling points(M-1 and M-4) to confirm organic acid elimination(<10 µg/m³).
Temperature/Relative Humidity data loggers(UX100-011, HOBO, USA)(accuracy within ±0.21°C and ±2.5% RH) were used for the entirety of the experiment to monitor the environmental conditions.
4. RESULTS
4.1. Pigment identification
The elements detected by XRF scanning and subsequent identification of pigments are listed in Table 1. The L*, a*, and b* values for each scanned area(sample) are provided for reference. Arsenic was the main element found in the yellow and green pigments(samples Ayel1, Ayel2, Byel1, Agre1, and Bgre1). The tanned yellow pigment on the right-side print also contained an iron compound(samples Ayel1 and Ayel2), and iron was identified even in the blue pigment(sample Bblu1) and in small traces in the green pigment of the middle print(sample Bgre1). Barium was identified in the red pigment(samples Ared1 and Ared2). No metal element was identified in the black pigment(sample Abla1).
The yellow pigment of the right-side print is believed to be a mix of orpiment(As2S3) and yellow ochre[FeO(OH)·nH2O] owing to the presence of As and comparatively higher concentration of Fe and also its visual appearance. It is a noticeably different tone of yellow than the yellow of the middle print, wherein only arsenic was identified. As Fe was detected, we believe that the green pigment was obtained by mixing orpiment and a small portion of Prussian Blue (C18Fe7N18), a common mixture. The red pigment is suspected to be lake carmine with a barium salt base owing to its appearance. The use of barium white as a base and extensor for red dyes in Japanese woodblock printing during the Meiji period was reported in 2018(Cesaratto et al., 2018). The black pigment is most likely made of organic material.
4.2. Preliminary measurement of organic acids
Organic acids measured in the storage room were found to be below the TPM’s recommended limit, which is approximately 63 µg/m3. It should be noted that this measurement was conducted in the month of December; when another measurement was performed in May, the quantity of organic acids had risen to 130 µg/m3.
Despite the optimal values in the storage room, we found a significantly higher concentration of organic acids inside the wooden box: approximately 3.8 times higher than the concentration in the storage room. The concentration inside the folded hōsho(measured at the fold) was less than half of the concentration inside the box(refer to Table 2 for results regarding storage materials). The listed values were all corrected for temperature to facilitate data assessment. Temperature and relative humidity conditions during sampling are also listed for reference.
Table 3 shows results obtained from measuring off-gassing from the two woodblock prints at six different sampling points representing different color areas. The L*, a*, and b* values for each sampling point are provided for reference. Concentrations between 10 and 63 µg/m³ were measured for the different sampling points. With the exception of the green area(sampling point M-05), the right-side print displayed considerably higher off-gassing compared to the middle print. The red area of the right-side print(M-06) exhibited the highest off-gassing, while the red area of the middle print(M-03) exhibited the lowest.
4.3. Measurement of organic acids in Phase 1 and Phase 2
Organic acid re-emission was observed after the follow-up periods in both phases of the experiment, as shown in Figure 7. The lowest off-gassing values corresponded to Phase 2 of the experiment as expected, although Phase 1 results also showed that mitigated emissions were maintained in the woodblock print. Nevertheless, compared to storage in a clean environment, the original wooden box storage condition seems to increase off-gassing from the woodblock print by 32±19%.
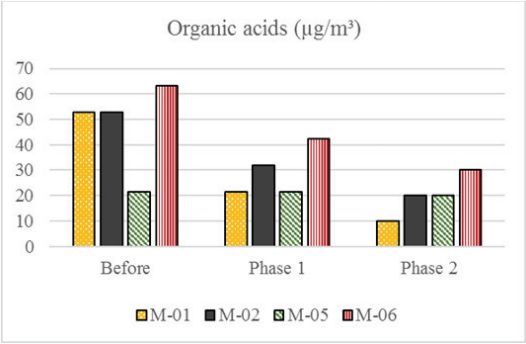
Comparison of the concentration of organic acids (µg/m3) in the off-gassing from four sampling points of the woodblock-print before, after Phase 1, and after Phase 2 of the experiment.
Variation in the emission concentrations between different sampling points continued to occur in Phase 1 and Phase 2. Organic acid reduction was observed in all color areas, with the exception of point M-05(green area), which maintained the same values throughout. M-01(yellow) displayed the biggest off-gassing reduction, while M-06(red) displayed the lowest.
Temperature and relative humidity(RH) information retrieved from the data logger showed that environmental conditions remained stable at optimal levels and with similar values for both phases of the experiment. No sudden drops or rises in temperature and RH values were observed. During the 20-day storage period(storage room) of Phase 1, the average(±standard deviation) temperature value was 18.5±0.5°C, with a maximum of 20.4°C and a minimum of 17.8°C, while the RH ranged from 48.7 to 52.7%, with an average value of 50.4±0.7%. For the 25-day storage period(clean air space) Phase 2, the temperature and relative humidity averages were 18.3±0.6°C and 54.1±1.2%, respectively. The maximum temperature peaked at 19.3°C, with a minimum of 17.7°C and RH ranged from 47 to 55.5%.
5. DISCUSSION
5.1. Storage environment
A somewhat organic acid-concentrated microclimate was found inside the wooden box that the ventilation system of the University of Tsukuba Library did not reach. It is known that wooden boxes historically used in document storage tend to emit moderate to high levels of organic acids, particularly acetic acid(Gibson and Watt, 2010), although the wood species more commonly used in Japanese storage materials (paulownia and Japanese cedar) have been noted to emit comparatively lower rates of acetic acid(Oikawa et al., 2005). Unfortunately, it was not possible to ascertain the wood species of the box. Nevertheless, we found these results to be similar to the results published by Fenech et al.(2010), who investigated VOC concentrations in European libraries and measured a concentration of acetic acid inside the archival boxes of ~70 ppb(approximately 175 µg/m3), about 3.5-times the concentration of ~20 ppb(approximately 50 µg/m3) measured in the repository room where the boxes were stored.
Comparing the preliminary measurement values with the Phase 1 and Phase 2 results, it seems clear that the main source of organic acid off-gassing in the right-side woodblock print is not endogenous but exogenous, i.e., the storage environment provided by the wooden box. The contrastingly low off-gassing levels of the middle print though suggest that the paper folder and the prints sandwiching it provide some level or protection by serving as obstacles to the migration of VOCs emitted from the box. It has been suggested in published research that acidic volatile compounds can migrate between stacked sheets of paper but tend to accumulate and become trapped in the sheets that are further away from “escape areas”-for instance, released organic acids from cellulose degradation would accumulate at the bottom and middle sheets of a stack, the upper part of which is open to the atmosphere(Carter et al., 2000). In this case, we suspect that the organic acids released from the wood box accumulate on the paper folder and the first print, and the remaining organic acids that managed to migrate through these obstacles may eventually become trapped in the paper folders that are stacked in the middle.
5.2. Influence of the color materials and degradation in off-gassing behavior
The VOC migration theory mentioned earlier might also apply to the relative location of the sampling point measured in the print, as the off-gassing values obtained from the right-side print were not uniform. In particular, sampling point M-05(green color area) constantly showed the same low off-gassing value in all steps of the experiment, and we suspect that this is because it is located in the corner of the print. The margins of a woodblock print are likely to be in a different conservation state than the rest of the print owing to human handling and proximity to the atmosphere.
Aside from point M-05, the re-emission trend displayed in the other sampling points suggests that pigment materials affect gas adsorption and desorption behavior. For instance, a comparison between Phase 1 and Phase 2 results reveals a certain consistency in the comparatively lower re-emission values of the yellow area, and the opposite happens in the red area. For the yellow area(M-01), we posit that the crystal topography of an orpiment layered area displays slow adsorption and low retention properties. Similarly, the comparatively higher off-gassing levels in the M-06 sampling point, observed in all steps of the experiment, hints that the red pigments tend to adsorb and retain volatile organic acids. However, upon further examination, we found that this reasoning does not explain the surprisingly low off-gassing detected in the red area of the middle print(point M-03), which is believed to contain the same pigments. It is possible that there is an additional endogenous source of off-gassing in the area surrounding sampling point M-06, such as an ongoing chemical reaction that is releasing organic acids as a by-product.
5.3. FFU system as a mitigation strategy
The FFU system can be used as an innocuous method to reduce VOC emissions from paper and wood materials. Although rinsed artifacts that are returned to storage boxes with high concentrations of VOC will exhibit an aggravated tendency for re-emission, Phase 1 results revealed that the rinsed woodblock print could still maintain mitigated values for a 20 day-period in its original storage conditions. Ideally, the storage materials should also be rinsed along with all the stored artifacts and accompanying folders to achieve longer permeance of results. Moreover, the FFU system can be especially useful in cleansing artifacts that emit high amounts of VOCs which therefore put other nearby collection items at risk.
From a practical viewpoint, however, the FFU system can be expensive as well as space- and time-consuming. Its installation is also not very simple, although modifications can be made to the methodology presented here. In addition, VOC elimination by an FFU system is only temporary as it cannot permanently eliminate the tendency of re-emission in organic materials. For long-term preservation, the FFU system should be combined with other mitigation measures to maintain low emission levels, such as using alkaline buffered sheets to help neutralize volatile acids. For woodblock print storage, because alkali materials pose an uncertain risk for prints with pH-sensitive dyes, they should only be used to cover the outer surface of the hōsho folder so that they do not come into contact with the prints. Another option would be to use molecular sieves, such as zeolites or activated carbon. There are limited studies on the application of molecular sieves to organic acid adsorption, but Grøntoft et al.(2015) found that activated carbon cloth could be used to significantly reduce organic acid concentrations inside showcases. The caveat here is the potential corrosive properties of the chloride present in activated carbon cloth; therefore, the application of these materials in cultural heritage pieces of art should be carefully considered.
6. CONCLUSION
We were able to ascertain organic acid emission values from different areas of a woodblock print and observe that the storage environment and storage practices(particularly the stacking order) seemed to significantly influence on the off-gassing behavior of these artifacts. The slight variation in acidic gases emitted from different areas of the print can be explained by differing VOC adsorption and retention properties of the color materials and possibly ongoing degradation processes as well. The FFU system was found to be an effective mitigation strategy for quick and temporary solutions; however, for long-term preservation, other methods of combating re-emission and storage microclimates should be considered.
Future research on VOC adsorption tendencies of different pigments could build on the assumptions presented in this paper regarding the off-gassing behavior of woodblock prints and other paper materials with color compositions. Having a deeper understanding of how pigments can trap and accumulate VOCs can reveal new insights on degradation factors in stored artifacts. In addition, more research on the issue of organic acids and other VOC-concentrated microclimates created by storage boxes is warranted, as well as on solutions to combat it. More investigation can certainly be performed on the efficiency of the FFU system as a mitigation strategy; for example, it would be interesting to see the results of a similar organic acid rinse experiment where the FFU is equipped with filters only targeting acidic gases, which could allow for non-acidic VOCs to be retained in the rinsed object as an alkali reserve.