Manufacturing Techniques and Alloying Compositions of Metal Decorative Artifacts in 18th Century, Myanmar
Article information
Abstract
Konbaung Dynasty was the last unified dynasty that ruled Myanmar from 18th to 19th century. During this time Buddhist art flourished in Myanmar due to the interest of the rulers toward their traditional culture. Metal decorative artifacts in the 18th century are classified into structures and Buddha statues. They are further subdivided into gilt-bronze and bronze objects, depending on their material component. Three-dimensional gilt-bronze decorative artifacts were cast with a brass alloy of Cu-Zn-Sn-Pb and their surfaces were gilded with extremely thin gold leaves (less than 1 μm in thickness). The gilded layer approximately comprised 10 wt% silver in addition to the main element, gold. The lack of Hg in the gilded layer, indicated that the amalgam gilding technique was not applied. The analysis results indicated that the lacquered gilding technique was applied to the objects. Bronze decorative artifacts without gilding were cast with materials containing Cu-Sn-Pb. The bronze pavilions and bronze Buddha staues were crafted using the same alloy of high-tin bronze, which approximately contained 20 wt% Sn. No heat treatment was applied to reduce the brittleness of the objects after they were cast with a large amount of Sn. The most significant difference between the gilt-bronze and bronze decorative artifacts lie in their elemental compositions. The gilt-bronze decorative artifacts with their gilded surface were manufactured using brass containing zinc, while the unplated bronze decorative artifacts were composed of bronze containing tin. Artifacts of the same type and size are classified differently depending on the materials utilized in the surface treatment such as gilding.
1. INTRODUCTION
The Konbaung Dynasty was the last dynasty that ruled Myanmar from 1752 to 1885. It created the second-largest empire in Burmese history and continued the administrative reforms initiated by the Toungoo Dynasty, laying the foundations of the modern state of Myanmar (Phayre, 1883, 1967 ed.). King Alaungpaya centralized the previously divided regions of Myanmar and established his rule. He was succeeded by King Hsinbyushin (1763∼1776) and King Bodawpaya (1782∼1819), who prominently contributed to developing the heritage of Myanmar dynamically from the mid-18th to early 19th century (Choi, 2006). In the times of King Bodawpaya, Konbaung Dynasty occupied Rakhine State, an area in the west of Myanmar, and attacked Siam (now Thailand) three times, culminating in its growing power. At this time, the people of the dynasty became interested in their traditional culture to counter the growing influence of Siamese culture, and this led to vigorous revivalism of Buddhism and construction of new temples and pagodas. However, the dynasty became embroiled in a war with Britain in the mid 19th century. This war seriously eroded the national power of the Konbaung Dynasty. King Mindon relocated the capital to Mandalay and attempted to repel the British invasion by keeping the people of Myanmar united through Buddhism (Busan Museum, 2019). In Mandalay, Buddhist art flourished under royal patronage and Mandalay became the biggest production area of Buddhist works of art in modern period, resulting in the birth of the Mandalay style, an important element of Myanmar Buddhist art. Though the Mandalay style was not created before the 19th century, vigorous revivalism of tradition and Buddhism in the 18th century served as a foundation for the Mandalay style. Accordingly, metal decorative artifacts used for Buddhist services in the 18th century play an important role as cultural heritage objects in connecting the traditions of Myanmar with the Mandalay style in the 19th century. The images of structures and Buddha were simplified for creating threedimensional metal decorative artifacts. Interestingly, metal decorative artifacts with similar shapes and sizes were made of different materials such as bronze and gilt-bronze. The major difference between them is the additional presence of gilding on the surface of gilt-bronze objects. However, as metal decorative artifacts are objects of worship in Buddhism, there is possibility that they might be made in different materials due to the different significance attached to the bronze color and gold color decorative artifacts. This study intends to determine the difference in the manufacturing techniques of bronze and gilt-bronze decorative artifacts of similar shapes and sizes through scientific analysis.
2. METHODOLOGIES
2.1. Sampling and Sample preparation
Four metal decorative artifacts from the Bagan Archaeological Museum in Myanmar were analyzed in this research (National Research Institute of Cultural Heritage, 2017). These artifacts have the material properties of bronze and gilt-bronze, and the basic specifications are summarized in Table 1. Samples were taken in the smallest size possible to snsure that, no damage was caused to the original shape of the artifacts. Sampling was carried out using a diamond circular saw blade mounted on an electric motor tool. After the collected samples were packed in plastic bags individually to prevent them from mixing with each other, the information about the object was recorded. The objects’ sufaces from which the samples were taken have been restored with epoxy resin to maintain the original shapes of the artifacts.
After checking the name and number of each artifact, samples were mounted in epoxy resin for analysis. The sample surfaces were polished smooth by using a polishing machine with polish papers in the order of serial number #800, #1000, #2000, to #4000, finishing with a 1 μm diamond suspension. The surfaces of the smoothly polished samples were etched in a solution of ethyl alcohol (120 mL), hydrochloric acid (30 mL), and iron chloride (Ⅲ) (10 g) for several seconds. After this process the samples were cleaned with water and dried.
2.2. Method of analysis
The microstructures of the samples were observed by using a reflected light microscope (DMRBE, Leica, DEU) to identify the manufacturing techniques applied to the metal artifacts. The entire microstructure was observed at low magnification (x25, x50) and specific region were observed at high magnification (x100, x1000). If a higher magnification (higher than x1000) beyond the capacity of the optical microscope was required, sample surfaces were carboncoated and observed by using a scanning electron microscope (JEOL JSM-IT300, JEOL, JPN).
Components of the entire sample were observed at low magnifications by using energy dispersive X-ray spectroscopy (JSM-IT300/Oxford, JEOL, JPN) attached to the scanning electron microscope and the photomicrographs were analyzed to identify the alloy components. The surface analysis was performed at least five times at low magnifications (x100 to x400) by using the scanning electron microscope and the results of the analysis were averaged. Clues that led to the understanding of the manufacturing techniques such as the presence of gold leaf, inclusions, lead particles and grain boundaries were observed at high magnifications. The sample surfaces were carbon-coated to give conductibility, and they were analyzed at an acceleration voltage of 20 kV at a working distance of 10 mm.
3. RESULTS OF ANALYSIS
3.1. Bronze pavilion
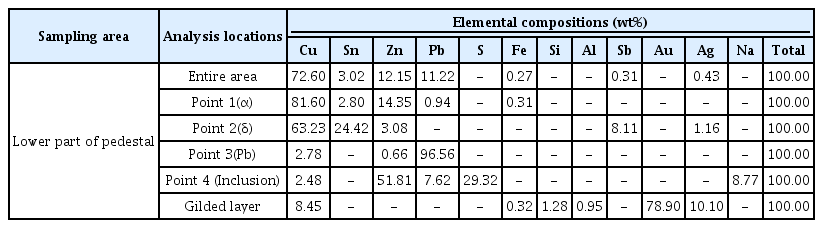
The first object considered for this study was a building-shaped bronze pavilion that was used for enshrining a bronze Buddha statue. The samples were taken from the base and edge of the structure and analyzed (Figure 1a). Figure 1b is a photomicrograph of the microstructure of the sample taken from the edge of the pavilion. It shows that the light grey α+δ eutectoid structure and dark grey lead are extensively distributed in the α phase formed in the background. Lead particles vary in size from fine ones to 80 μm. Figure 1c is an electron micrograph of part of Figure 1b, displaying non-metallic inclusions formed around the lead particles. Different phases such as the α phase and δ phase ard observed. Along with the lead particles, these phases were analyzes by SEM-EDS (Table 2). Point 1 in Figure 1c is the α phase. The α phase is a solid solution of copper and tin, and it contains 14.13 wt% tin. Point 2 is the δ phase and it contains 33.02 wt% tin. Point 3, which consists of lead particles, contains about 98 wt% lead and traces of copper. Point 4 indicates non-metallic inclusion, consisting mainly of zinc and sulfur with small amounts of copper, iron and sodium. Figure 1d, which is a photomicrograph of the microstructure of the sample taken from the base of the bronze pavilion, shows that it has the same microstructure as that of the sample taken from the edge of the pavilion, suggesting that the artifact was cast as a single piece. Figure 1e reveals the re-precipitated copper from corrosion. It was determined that casting was employed for shaping the pavilion, such that heat treatments including hot working and quenching was not required after casting.

Microstructure of the bronze pavilion; (a) Appearance of the bronze pavilion, (b) Microstructure of the edge of the pavilion, (c) Magnified lead particles and non-metallic inclusions (SEM image), (d) Microstructure of base of the pavilion, and (e) Magnified copper particles formed by corrosion.
3.2. Gilt-bronze pavilion
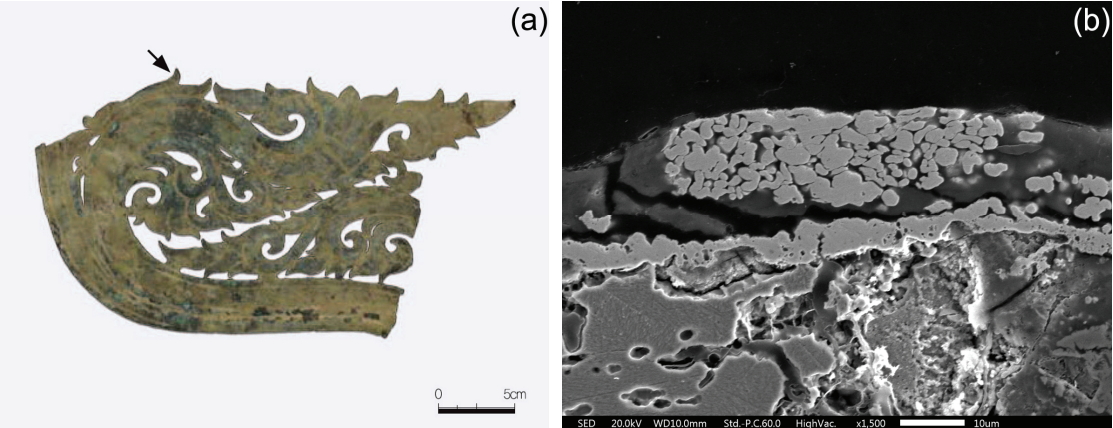
The second object considered for this study was a building-shaped gilt-bronze pavilion used for enshrining a gilt-bronze Buddha statue. The sample was taken from the gilded layer at the base of the pavilion and analyzed (Figure 2a). Figure 2b is a photomicrograph of the entire microstructure. It shows that the light grey α+δ eutectoid structure and dark grey lead are extensively distributed in the α phase formed in the background. Lead particles vary in size from fine ones to 150 μm. Figure 2c is a magnified electron micrograph of portion of Figure 2b, revealing the non-metallic inclusions formed around the lead particles here, different phases such as the α and δ phases can be observed. Along with lead particles, phases were analyzed by SEM-EDS (Table 3). Point 1 in Figure 2c represents the α phase, it comprises a solid solution of copper, zinc and tin, and it contains 14.94 wt% zinc and 3.13 wt% tin. Point 2 represents the δ phase, that contains 25.84 wt% tin. Point 3, which consists of lead particles, contains about 88.72 wt% lead and traces of copper and zinc. Point 4 indicates non-metallic inclusions, which consist mainly of zinc and sulfur with small amounts of iron and sodium. Figure 2d is the magnified electron micrograph of the gilded layer, it shows that the gilded layer as thin as 1 μm or less. An empty space and corrosion product is detected between the base metal and gilded layer, indicating that the base metal and gilded layer are not close to each other at a molecular scale. The gilded layer is composed of 63.36 wt% gold, 7.93 wt% silver and 23.99 wt% copper. Mercury was not detected in the gilded layer, this suggested that the amalgam gilding technique was not applied to the object (Figure 2e). Casting was employed for shaping the object to avoid the use of heat treatments, such as hot working and quenching at high temperatures.

Microstructure of the gilt-bronze pavilion; (a) Appearance of the gilt-bronze pavilion, (b) Microstructure of base of the pavilion (pointed out with an arrow), (c) Magnified lead particles and non-metallic inclusions (SEM image), (d) Gilded layer of the gilt-bronze pavilion, and (e) Enlarged view of gilded layer.
3.3. Bronze Buddha statue
The third object considered for this study was Buddhashaped bronze artifact and the sample was taken from the base of the pedestal and analyzed (Figure 3a). Figure 3b is a photomicrograph of the entire microstructure. I shows that the light grey α+δ eutectoid structure and dark grey lead are extensively distributed in the α phase formed in the background. Lead particles vary in size, ranging from few micrometers to 200 μm. Figure 3c represents the magnified electron micrograph of portion of Figure 3b. It reveals non-metallic inclusions formed around the lead particles. Different phases such as the α phase and δ phase ard observed. Along with lead particles, the phases were analyzed by SEM-EDS (Table 4). Point 1 in Figure 3c is the α phase. The α phase comprises a solid solution of copper and tin, and contains 13.36 wt% tin. Point 2 is the δ phase, contains 32.97 wt% tin. Point 3 consists of lead particles, and contains about 94 wt% lead and traces of copper. Point 4 indicates non-metallic inclusions, consisting mainly of zinc and sulfur with small amounts of iron, copper and sodium. As indicatedfor the above-mentioned objects, casting was employed to shape the object in order to avoid high-temperature heat treatments.

Microstructure of the bronze Buddha statue; (a) Appearance of bronze Buddha statue, (b) Microstructure of base of the Buddha statue (pointed out with an arrow), and (c) Magnified lead particles and non-metallic inclusions (SEM image).
3.4. Gilt-bronze Buddha statue
The fourth object considered for this study was a Buddha-shaped gilt-bronze artifact. The sample was taken from the gilded layer at the base of the artifact and analyzed (Figure 4a). Figure 4b is a photomicrograph of the entire microstructure. It shows the light grey α + δ eutectoid structure and dark grey lead are extensively distributed in the α phase formed in the background. Lead particles vary in dimensions and are observed to be up to 50 μm in size. Figure 4c is the magnified electron micrograph of a part of Figure 4b. It reveals non-metallic inclusions formed around the lead particles. Different phases such as the α and δ phases ard observed. Along with lead particles, the phases were analyzed by SEM-EDS (Table 5). Point 1 in Figure 4c represents the α phase. The α phase consists of solid solution of copper, zinc and tin, while containg 14.35 wt% zinc and 2.80 wt% tin. Point 2 represents the δ phase, containing 24.42 wt% tin. Point 3 consists of lead particles and contains approximately 96.56 wt% lead and traces of copper and zinc. Point 4 indicated non-metallic inclusions, consisting mainly of zinc and sulfur with small amounts of copper and sodium. Figure 4d is a photomicrograph of the gilded layer. It shows that the gilded layer is as thin as 1㎛ or less and that the gilded layer is separated from the base metal. The gilded layer is contains of 78.90 wt% gold, 10.10 wt% silver and 8.45 wt% copper. As the phtograph did not detect mercury in the gilded layer (Figure 4e). Casting was employed for shaping and heat treatment for changing the shape such as hot working and quenching at high temperature after casting was not carried out.
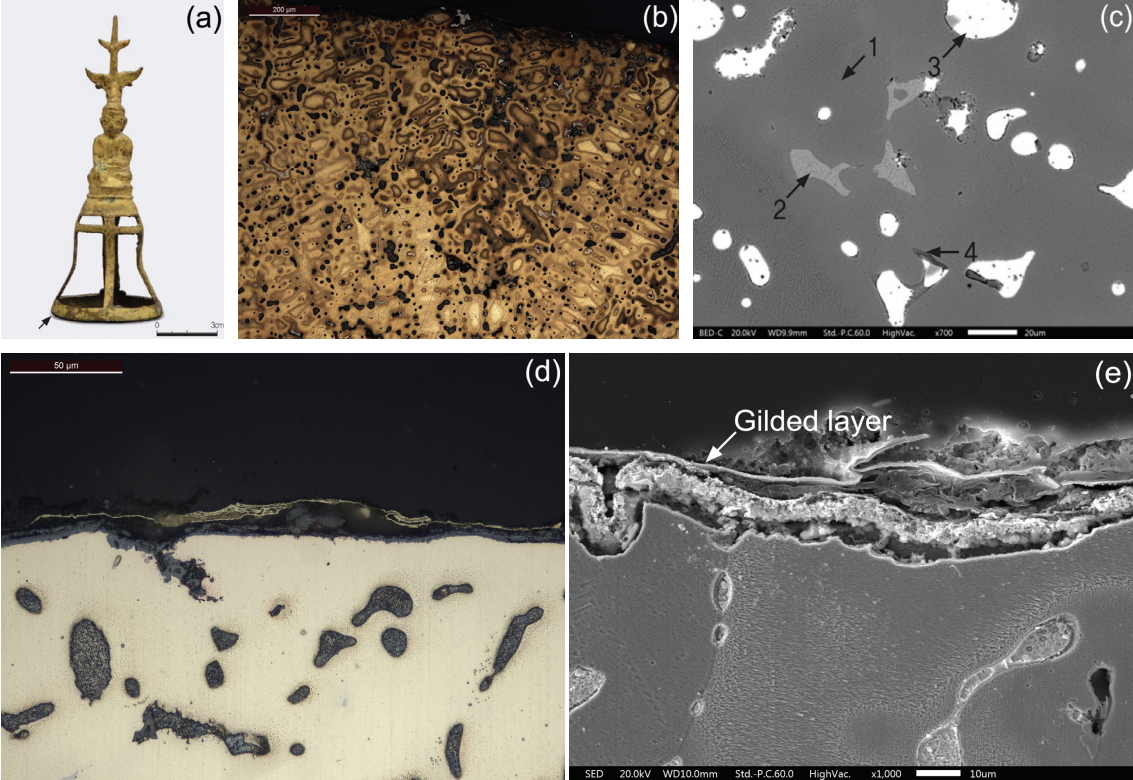
Microstructure and gilded layer of the gilt-bronze Buddha statue; (a) Appearance of the gilt-bronze Buddha statue, (b) Microstructure of base of Buddha statue (pointed out with an arrow), (c) Magnified lead particles and non-metallic inclusions (SEM image), (d) Gilded layer of the gilt-bronze Buddha satue, and (e) Enlarged view of gilded layer (SEM image).
4. CONSIDERATION
Mercury was not detected in the gilded layer of gilt-bronze artifacts. This indicated that the amalgam gilding technique was not applied to the artifacts. The detected main elemental composition of the alloy for the base metal of gilt-bronze artifacts was Cu 72.60 wt%-Zn 12.15 wt%-Pb 11.22 wt%-Sn 3.02 wt%. It is difficult to make direct comparision, but the gilding on copper alloy with Cu 85 wt%-Zn 13 wt%-Sn 2 wt%, was tested for amalgam gilding. It showed that the gold amalgam did not attach to the base metal better than copper. The gold flowed down during testing and it was found that the gilding condition was not uniform and neat (Mun, 2004).
These results suggested that the technique of lacquered gilding might have been applied to the metal artifacts in Myanmar in the 18th century. In Myanmar, the technique of fabricating lacquerware was highly developed during this time as it is the place of origin of Melanorrhoea usitata (known as Burmese lacquer) and the gilding technique with lacquer also existsed in Myanmar during this period. As lacquer was readily available, it was used for painting metal and wood works. Thus, there is a high possibility that the lacquered gilding technique was applied to the metal artifacts of this period. On the other hand, this possibility can lead to the consideration of lacquered gilding as a typically traditional technique. Thus, the scientific analysis results of the gilt-bronze of the 18th century were compared wit those of the gilt-bronze decorative artifacts objects excavated from Bagan. Figure 5a is a floral gilt-bronze artifact that was manufactured in the 13th century, Bagan period. The floral gilt-bronze artifact is carved in openwork with flower patterns with its front surface being gilded. Figure 5b is the magnified photomicrograph of the gilded layer, showing the uneven thickness of the gilded layer and the grain particles. The microstructure observed in Figure 5b was analyzed by EDS and summarized in Table 6. The base metal contains 97.39 wt% copper and traces of arsenic (Table 6). Gold and mercury were detected as main components in the gilded layer. This suggests that amalgam gilding was applied to one side of the decoration plate made of copper. In addition, as the gilded layer found to be uneven, and gold particles in the form of grains are easily observed, this indicates that gold powder was most likely used for gilding. Though gilt-bronze artifacts of the 18th century were manufactured about 500 years later than the floral gilt-bronze artifact that was subjected to the amalgam gilding technique, the correlation between them can be examined from a point that both the artifacts have something in common with a period of rule during unified dynasties by the Burman. Consequently, it can be presumed that the amalgam and lacquered gilding techniques might have been coexisted, and each gilding technique was applied according to the shape and utility of the objects.
Microstructure and gilded layer of the floral gilt-bronze artifact; (a) Appearance of the floral gilt-bronze artifact, and (b) Enlarged view of the gilded layer (SEM image).
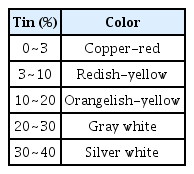
The bronze decoration without gilding represents the high-tin leaded bronze, which contains a large amount of Sn, this alloy is a completely different bronze alloy as compared to the gilt-bronze alloy. As this alloy comprises more than 20 wt% Sn along with copper, its color is extermely similar to bright yellow (Table 7) (Huh, 2006). In particular, the more the addition of lead, the brighter is the surface color of the alloy, this is because a large amount of the δ phase is formed in this method as compared to that observed for the Cu-Sn (20 wt%) alloy. The purpose and signficance of using of bronzeware is directly associated with its color. The alloy of Cu with 20% Pb shows a similar to that of pure copper, while the alloy of Cu with 20% Sn shows a lemon yellow color. The bronze alloy of Cu-Sn changes its color according to the percentage of Sn. Therefore, understanding the elemental compositions of bronzeware was crucial during the 18th century for analyzing technical properties such as melting temperature, hardness and color (Kang, 2006). The bronze alloy of Cu-Sn is susceptible to breakage due to the brittleness caused by a high Sn percentage in the δ phase (Scott, 1991). The bronze artifacts of pavilion-shaped structure and Buddha statue have something in common as their casting metal contains approximately 20 wt% Sn. This indicates that they have the same alloy composition and similar manufacturing techniques were applied to both the objects. In particular, the high percentage of Sn in the alloy of both the artifacts suggests that surface colors were more significant to people in the 18th century as compared to the possibility of short-lasting objects; this is because brittleness Sn increased. In other words, bronze artifacts were manufactured to obtain bright gold color from high-tin bronze. In addition, it is presumed that the alloy composition of Cu with 20 wt% Sn and 10 wt% Pb was the most appropriate alloy for developing bronze metal artifacts without gilding at that time.
5. CONCLUSION
Metal artifacts, depending on their type, are classified into structures and Buddha statues. They are also subdivided into gilt-bronze and bronze objects based on their material composition.
1. Three-dimensional gilt-bronze decorative artifacts were cast with a brass alloy of Cu-Zn-Sn-Pb and the surfaces were gilded with an extremely thin gold leaf with thickness of less than 1 μm. The gilded layer is composed of about 10 wt% silver in addition to gold, which is the primary element. Mercury was not dotected during gilding, thereby indicating that the objects were not subjected to the amalgam gilding technique.
2. Bronze decorative artifacts without gilding were cast with materials consisting of Cu-Sn-Pb. The bronze pavilion-shaped structures and bronze Buddha statues exhibit similar features as that of high-tin bronze meaterials that contain approximately 20 wt% tin in their material composition, thereby resulting in the presence of a large amount of brittle δ phase in the microstructure. In the case of a high-sided bronze plate containing a similar percentage tin, the plate can be quenched to transform the δ phase into another microstructure. however, such a microstructure that is formed by quenching is not observed in the 18th century bronze decorative artifacts. This means that no heat treatment was applied to the objects to reduce brittleness after they were cast in an alloy with a large percentage of tin.
3. The most significant difference between the gilt-bronze and bronze decorative artifacts lie in their elemental compositions. The gilt-bronze decorative artifacts with their gilded surface was manufactured with brass containing zinc as the basic material while the unplated bronze decorative objects were made of bronze containing tin. Artifacts of the same type and size are classified differently, dependingon the materials utilized in surface treatment such as gilding. The non-metallic inclusions commonly in both gilt-bronze and bronze decorative objects are sulfide containing above 50 wt% zinc.
Acknowledgements
This study was supported by the National Research Institute of Cultural Heritage (NRICH) as a part of the Cultural Heritage Research & Development program. We are deeply grateful for their administrative and financial support.