|
 |
- Search
| J. Conserv. Sci > Volume 38(1); 2022 > Article |
|
ABSTRACT
Colour consistency is an important consideration when selecting pigments used on works of art. In this study, we analyse the colour difference between two sets of synthetic blue glass pigments acquired at least 8 years apart from the same manufacturer in Japan. The old pigment set (unused, dry powder with four different grain sizes) appears faded compared to the new set. These pigments are made available for artistic use, commonly in Nihonga or Japanese paintings. Raman spectroscopy and SEM-EDS results characterize these pigments as cobalt aluminate spinels dissolved in leaded glaze, a special class of complex coloured inorganic pigments that is not well-understood in the field of conservation. Colour difference between the old and new pigments with four different grain sizes were quantified by analysing photomicrographs with image analysis software. Blue pigments with coarse and extremely fine grains showed significant colour change compared to pigments with medium and fine grain sizes. The high occurrence of crystallites in the finer grains give a final colour that is bluer and lighter. Possible causes for the colour difference including manufacturing methods and storage environment are discussed.
Blue is a precious colour prized for its rareness in nature and it is often of high interest to the artistic field. Amongst the inorganic blues used on works of art, the family of cobalt spinel pigments is an interesting group for study because of its complex chemistry and wide variety of colours (Yoneda et al., 2016; Llusar et al., 2001; D’Ippolito et al., 2015). The earliest natural cobalt-based pigment, smalt, is a ground potassium glass containing cobalt that is known to be unstable and prone to discolouration. Studies have attributed the fading blue in smalt to be caused by the leaching of potassium cations, thereby shifting the cobalt ions from tetrahedral to octahedral coordination, resulting in the loss of blue hue (Robinet et al., 2011; Cianchetta et al., 2012). A more stable and lightfast cobalt blue pigment (Thenard’s blue or cobalt aluminate) was synthetically developed in 1802 and commercially produced for artistic use a few years later in 1807. Today, cobalt blue spinel pigments are available in other variations, including cobalt chromite blue-green spinels Co(Al,Cr)2O4 (PB36, The Color of Art Pigment Database, 2021) and cobalt chromium zinc aluminate spinels Co,Zn (Al,Cr)2O4 (PB36:1, The Color of Art Pigment Database, 2021) marketed as cerulean blue (Angelin et al., 2017). Methods of synthesis for cobalt blue are also changing, notably to reduce the expensive and toxic cobalt while maintaining an intense blue colour (Tang et al., 2017; Yoneda et al., 2018).
Pure cobalt blue is a spinel crystallized in a cubic lattice of AB2O4 stoichiometry (i.e. A = Co and B = Al), whereby Co ions are in tetrahedral coordination and Al in octahedral coordination. However, the exact composition is not consistent and can give rise to confusion in the literature data for Raman spectroscopy (Bouchard and Gambardella, 2010). The crystalline lattice may accommodate minor amounts of other elements (not specified in the basic formula) or the stoichiometry varies with changes in percentages of different metal oxides. For example, metal oxides in cobalt blue pigments can occur either as separate phases (e.g. Co3O4, Al2O3, SiO2, TiO2) or solid solutions (e.g. Co2SiO4 (olivine), Zn2SiO4 (willemite) and Co3−sAlsO4 (cobalt spinel, s = 0, 1, 2 and 3)). Industrially, this family of pigments made by solid-state reactions is often termed as complex inorganic-coloured pigments or CICPs. Metal oxides are strongly heated at high temperatures to form a solid solution as final product. As metal ions are locked into a crystalline lattice, the resultant pigment becomes highly durable, lightfast, and stable against the detrimental effects of chemicals and heat (Comstock, 2016). CICPs were developed originally for colouring ceramic bodies and glazes, glass matrices, and porcelain enamels, and today they are widely applied in plastics and paints (Comstock, 2016). These pigments, though readily available in commercial artists’ supplies, have not been meaningfully studied in the fields of studio art and art conservation.
While developing a “Blue Pigment Reference Analytical Database” at the Heritage Conservation Centre (HCC) in collaboration with the Shaanxi Institute of Preservation of Cultural Heritage (SIPCH), we came across two identical sets of blue glass pigments sourced from the same manufacturer in Japan that exhibited a difference in colour. The old set was acquired before the year 2010 and the new set later in the year 2018, both of which were housed at the Heritage Conservation Centre. As perceived by the eye, the old pigments appeared to have paler blue hues compared to the new pigments of the same grain size. This colour difference appeared more pronounced in the pigments with smaller grain sizes. These blue pigments were labelled as “Shin Iwa Enogu Gunjo (新岩絵具 群青)” of the “Asuka” series (Shin Iwa Enogu, 2021). “Shin Iwa” (artificial) pigments are marketed to end users as “new mineral pigment” or “artificial stone”. Its method of production was described to end users as follows (Pigment Lab, 2021): “First, we bake glaze used in pottery and lead glass to make artificial stone. Shin-Iwa is produced by grinding these ingredients in the same process as making mineral pigments.” According to the manufacturer’s description, the Shin Iwa Enogu production method involves dissolving metal oxides in a glaze constitute heated at 800-1000℃. The cooled solid is pulverized using a coarse crusher, then sifted and sorted according to its grain size by the ‘levigating’ process (Nakagawa Gofun Enogu, 2021b). Higher number denotes a smaller grain size (Particle Grades, 2021) and has a brighter blue. Though named as (Gunjo 群 青), which is known as azurite in Japanese context or as ultramarine by direct translation, its chemical composition is neither of these. The pigment is a cobalt blue spinel in a glaze material and is an example of CICP. Such naming discrepancies from the pigment’s chemical composition is very common amongst artistic pigments, especially with Chinese and Japanese suppliers (Chua et al., 2018). The blue pigment composition is made of 34.0 wt% SiO2, 29.0 wt% PbO, 30.0 wt% Al2O3 and 2.5 wt% CoO according to the manufacturer’s material safety data sheet (MSDS) (Nakagawa Gofun Enogu, 2021b).
These blue pigments are generally used for Nihonga or Japanese paintings, and possibly also used in acrylic-based paints or aqueous-based paints such as watercolour for artistic, restoration or architectural work (Nakagawa Gofun Enogu, 2021b). Nihonga (日本画) is a term first used in 1868 for traditional Japanese paintings to distinguish them from western style paintings. Today, Nihonga has evolved with western influences and does not strictly refer to a defined style of art. The practice generally involves the use of mineral pigments (ground powder) mixed with gelatine or animal glue binder (nikawa 膠) for the paint, and the use of substrates like wood, hemp, silk, and paper. Mineral pigments produced by the Japanese manufacturer for Nihonga are comprised of three different groups: natural (Tennen Iwa 天 然岩), artificial lead-containing glaze (Shin Iwa 新岩) and new lead-free glaze (Kyojyo Iwa 京上岩) pigments (Nakagawa Gofun Enogu, 2021b). The latter two synthetic groups are produced in a wide array of shades and consistencies that are not obtainable naturally. The old and new pigments in our current study belong to the artificial lead-containing glaze (Shin Iwa 新岩) group (Shin Iwa Enogu, 2021; Nakagawa Gofun Enogu, 2021a).
It is uncommon to find inorganic pigments in unused and dry powder form to discolour with age, which prompted our analytical investigation. In this paper, the colour differences observed in the “Shin Iwa Enogu Gunjo” cobalt blue spinel pigments from old and new sets were investigated. A total of 8 blue pigments were analysed with scanning electron microscopy (SEM) - energy dispersive spectroscopy (EDS), confocal laser scanning microscopy (CLSM) and Raman microscopy, where each set contains 4 blue pigments: Gunjo 7 (coarse), Gunjo 9 (medium), Gunjo 11 (fine), and Gunjo 13 (extremely fine). To compare the colour measurements of the old and new sets objectively, quantification of colour difference and grain size differences were also analysed from digital photomicrographs using image analysis software.
Old and new blue pigments with various grain sizes from the manufacturer (Nakagawa Gofun Enogu, Kyoto, Japan) were analysed in this study. The blue pigments were classified as “Shin Iwa Enogu Gunjo (新岩絵具 群青)” synthetic pigments of the Asuka series. The old set was purchased before 2010 (approximate year of purchase: 2007-2009) and the new set in the year 2018. Both pigments came in packaged sets of small, sealed glass vials; the old set was originally kept in a wooden cabinet and the new set in a metal cabinet, both housed in an air-conditioned conservation lab at the Heritage Conservation Centre, Singapore.
Gunjo 7, 9, 11 and 13 were studied. The number indicates grain size. The larger the number, the smaller the pigment grain size (Particle Grades, 2021). The numbers 7, 9, 11, 13 refer to coarse, medium, fine and extremely fine grains respectively.
Hitachi SU5000 Scanning Electron Microscope (SEM) with a Bruker Energy-dispersive x-ray spectroscopy (EDS) detector was used to examine the pigment particles on carbon tape. Partial vacuum was set at low pressure of 50-60 Pa and SEM images were obtained using a backscattering detector with 10 kV accelerating voltage. The images acquired using the backscattered electron (BSE-ALL) mode that encompass both composite and topography information. The elemental composition and mapping of the blue pigments were obtained at a working distance of 10 mm and 15-20 kV accelerating voltage.
The old and new pigments were examined at nanometre level with a non-contact, high resolution (Lext OLS 4100, Olympus, Japan) 3D measuring laser microscope. Fully focused images were acquired using a 405 nm laser source scanned across the depth of the grains with 0.06 µm pitch, collected on a 16.6M pixel scan CCD camera. Objective lens 50 x (N. A. = 0.95) and 100 x (N. A. = 0.95) were used for Gunjo 7, 9 and 11, 13 respectively. Brightness and focus were adjusted manually. All images obtained were not further processed or corrected.
Raman measurements were obtained using a Invia Qontor Renishaw confocal Raman Microscope, equipped with a Leica DM2700M optical microscope. Pigment powder was placed on a clean glass slide for analysis. Raman spectra were acquired with a 532 nm laser, using a 100 x objective lens, 1800 l/mm diffraction grating and a Peltier-cooled CCD array detector. A 5% laser power with 3 s exposure and a minimum of 8 spectra scans were accumulated between 120-1800 cm-1 . For each pigment sample, at least 5 random grains were selected for analysis, whereby Raman spectra were acquired from a blue spot and white spot on each grain to account for any heterogeneity of the pigment. No baseline correction or data post-processing was done.
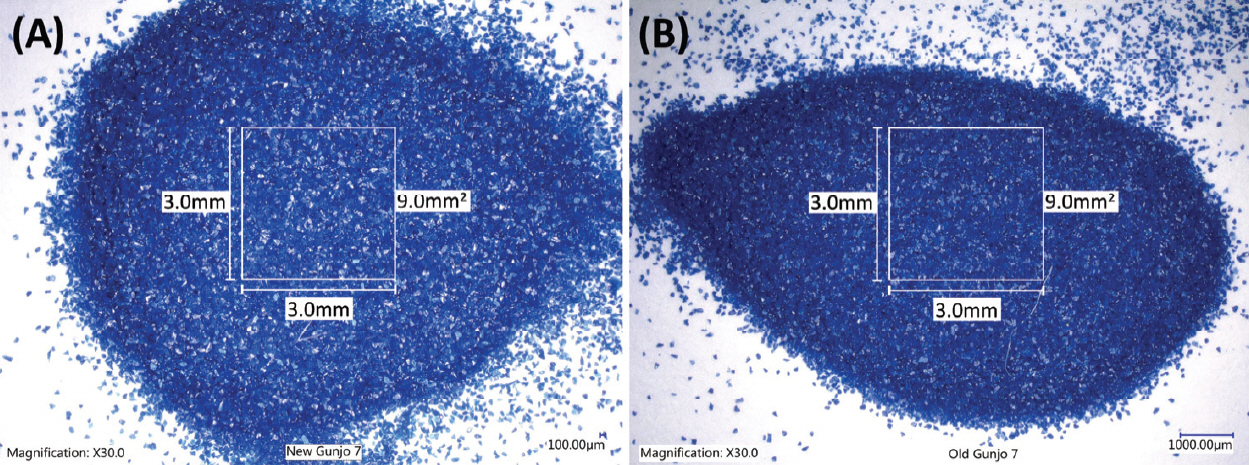
For imaging colour and grain size of the blue pigments, an optical microscope (Keyence digital microscope VHX6000 with VH-Z20R zoom lens (20 x – 200 x zoom range) from Japan) was used. 30 x magnification was mainly used to image wide areas (significantly wider than 3.0 mm x 3.0 mm (9.0 mm2) in area) of pigments. Figure 1 shows the optical photomicrographs of the old Gunjo 7 and the new Gunjo 7 pigments on white papers.
Image analysis software (PicMan from WaferMasters, Inc., CA, U.S.A.) was used for digital image analysis of the colour of individual grains and the average colour of an aggregate of grains and dimensions such as length, area, and grain size. PicMan can read RGB brightness information on individual pixels from any digital image file and convert colour information in HSV, L*a*b*, Munsell colour space and provide a corresponding hexadecimal colour code. It also generates statistical analysis data for the region of interest (ROI) for easy comparisons.
PicMan software has been used for automatic analysis of digital images in semiconductors, materials science, nanotechnology, food industry, biology and medical image analysis including colorimetric applications. Applications for use in artifact conservation and cultural heritage restoration have been reported in recent years. Activities included a study of paper foxing, colour analysis, image comparison and damage mapping examples (Kim et al., 2019a; Yoo et al., 2019; Yoo and Yoo, 2021; Kim et al., 2019b; Kim et al., 2021; Lee and Wi, 2021; Yoo et al., 2021).
The blue pigments with four different grain sizes from the old and new sets were characterized with SEM-EDS, CLSM and Raman spectroscopy. The colour differences between the old and new sets were determined from optical photomicrographs using image analysis software to investigate possible causes.
The surface morphology of the old and new pigments with four grain sizes was examined with SEM (Figure 2). The pigment particles appear rock-like with glassy striations. Interestingly, in the old pigment batch, distinct polygonal crystallites about the size of 1 μm were spotted on the surface of pigment particles. These crystallites are more densely crowded in smaller pigment grain sizes and are usually of hexagonal shapes. Although some bright particles also appear in the new pigments, they are usually small and irregularly shaped and do not pose much concern. When the old pigments were probed with EDS, these crystallites mapped out a higher concentration of lead (Pb). An example of elemental composition of old Gunjo 13 is shown in Figure 3. At this point, it is not clear that such lead-based crystallites are new growths or were originally present in the pigment.
Both old and new pigments showed the same elemental composition that corresponds well to the chemical data listed in the MSDS. The dark spots in old and new pigments correspond to cobalt (Co) and aluminium (Al) which contribute to the blue chromophore, and sometimes show traces of chromium (Cr) and/or zinc (Zn) that alters the shade of blue. These elemental oxides are embedded in a glassy Pb/Si matrix; therefore, the pigment is characterized as a cobalt aluminate dissolved in leaded silicate glaze. As these Japanese blue glass pigments show striations of the glass and contain cobalt and aluminium, they may be mistaken as smalt (potassium glass containing cobalt) or pure cobalt blue without proper analysis.
Using light microscopy, the pigment particles were observed to be glassy with a blue hue and contain white spots that appear as bright tiny particles. The blue hue in the glass is contributed by the cobalt ion chromophore. The old pigments show more white particles than the new pigments and this difference is particularly evident in pigments of smaller particle sizes (Gunjo 11 and Gunjo 13). An example of old and new Gunjo 13 is shown in Figure 4.
The Raman laser was focused on the blue spots (i.e. glassy matrix) and white spots (i.e. tiny particles or crystallites). The Raman spectra of the blue spots in both old and new Gunjo pigments, regardless of particle size, correlates well to a cobalt blue pigment (Figure 5). Two strong bands hovering around 200 and 510 cm-1, and two weak bands around 405 and 650 cm-1, are typical of a cobalt aluminate spinel. The peak maxima do vary with a few wavenumbers, and this can be attributed to the nature of spinel pigments: (1) symmetry distortion in spinel cubic structure, (2) different percentage of other ions in a solid solution (Bouchard and Gambardella, 2010). It is likely that the Gunjo pigment takes the form of the Co/Al based spinel (CoxAl3−xO4), which is the most common spinel form analysed in another Raman spectroscopy study of industrial cobalt blue spinels used in the field of art (Bouchard and Gambardella, 2010).
Unlike pure cobalt blues, these pigments also produce a strong Raman band at 1045-1048 cm-1, which is particularly intense in the white spots. It is detected intensely in all the white spots of the old pigments. In the new pigments, it is less frequently detected and if seen, it appears as a weak band or only occasionally as an intense band (Figure 5). It is found that the band is more intense and readily detected in smaller particle sizes where the white spots are more densely crowded in the smaller particles. The Raman spectrum of the crystallite is attributed to lead hydroxycarbonate (hydro cerussite) or similar carbonate form. This is also supported by the detection of lead in the crystallite with SEM-EDS. Lead hydroxycarbonate shows an intense band at 1050 cm-1 in the Raman spectrum and the difference in peak position (within 5 cm-1) in the crystallite may be due to the differences in crystal structure with respect to its molecular environment, in this case the glassy matrix.
According to the MSDS of the pigments (Nakagawa Gofun Enogu, 2021b), lead hydroxycarbonate is not originally present in the composition. Also, it is unlikely that a metal carbonate is included in the formulation of metal oxide solid solutions. Furthermore, the crystals occur in random clusters on the pigment grains that do not seem to be an intentional adulteration. Hence, we deduce that the lead hydroxycarbonate crystals are new natural growths that stem from lead oxide in the pigment. It is uncertain how these crystallites appear in the old pigments and whether this phenomenon will reoccur in the new pigments. It was reported that lead oxide forms lead hydroxycarbonate upon interaction with carbon dioxide and water vapour in the air (Black and Allen, 1999). Atmospheric corrosion of lead was strongly accelerated by trace amounts of acetic acid vapour, which can originate from wood. Acetic acid vapour can react with lead or lead oxide to form lead acetate, an essential precursor to lead hydroxycarbonate (Niklasson et al., 2008). The old pigments were previously stored in a wooden cabinet and the emission of acidic vapours from the wood could be one reason for promoting the crystallite growth. We also note that the old pigments are stored in glass vials that are screw capped and the usage was low to negligible since acquired into the reference collection. Such undisturbed storage environment could promote crystal growth.
In another unpublished study, we also analysed 35 blue pigments that were also sourced from Japan and their compositions are all spinels in leaded glaze (mainly cobalt-based). These pigments were procured approximately in the years 1970-1979 (Hoevel, 2021), which coincides into the Shōwa period (1926-1989) when the development of new mineral pigments in Japan increased exponentially (Pigment Lab, 2021). These leaded glaze pigments, though very old in age, do not show distinct crystallites as are encountered in the old set in this study. This seems to suggest that the crystal growth encountered in this study is not a consequence of inherent ageing or degradation of leaded glaze pigments.
In the field of art conservation, lead hydroxycarbonate, also commonly known as lead white, is the principal white pigment used in paintings and ceramic glazes from ancient times until the 20 th century (Smith et al., 2002). In classical times, lead white was prepared synthetically by exposing lead to vinegar (acetic acid) vapor (Gonçalves et al., 2010). It is interesting that our current results show lead white manifesting itself as crystallite growth in aged modern spinel pigments. The paler shade observed in the old pigments is probably due to the reflection from lead white crystallites. Lead white is also known to darken when exposed to sulfidic gases or other sulphur-containing pigments, resulting in works of art losing brilliance (Smith et al., 2002). It is worthwhile to highlight that at the point of SEM-EDS analysis, sulphur or consequential darkening was not observed in the old pigments. The sensitivity of the detection of sulphur in the lead-based pigment can be limited due to the close X-ray energies of sulphur and lead.
Figure 6 shows 3.0 mm x 3.0 mm (9.0 mm 2) areas of 30 x magnification optical photomicrographs of the Gunjo pigments with four different particle sizes, obtained before 2010 (old) and 2018 (new), under the same illumination conditions. Grain size becomes smaller as the number increases (i.e., 7 (coarse), 9 (medium), 11 (fine) and 13 (extremely fine)) (Particle Grades, 2021). Generally, the colour of blue pigment from the same material became lighter as the grain size decreased. Colour differences between old and new pigments with coarse particle size (suffix 7) and extremely fine particle size (suffix 13) are easily noticeable.
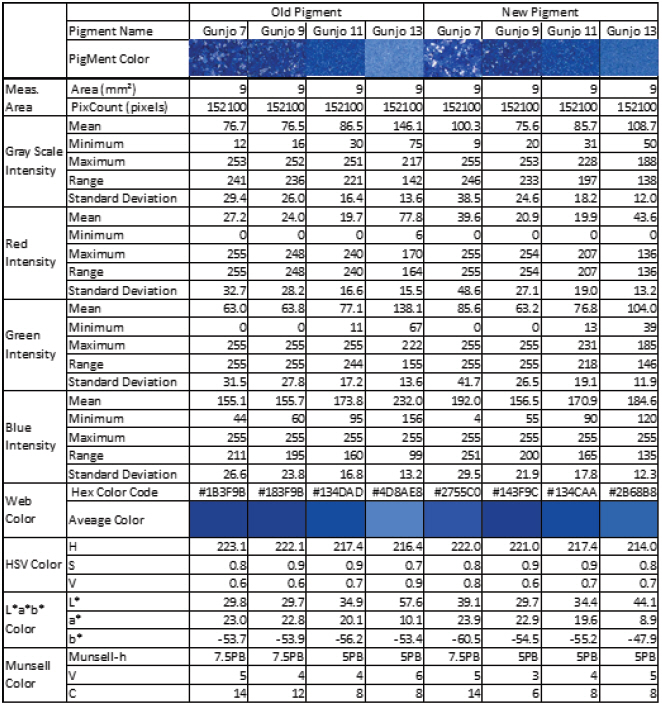
The colour of old and new Gunjo pigments with different grain sizes, in 3.0 mm x 3.0 mm ROIs, were analysed using image analysis of optical photomicrographs shown in Figure 6. RGB colour information of entire pixels (152,100 pixels) in each ROI were extracted and statistically analysed. Average, minimum, maximum, range, standard deviation of RGB and grayscale intensity were calculated. Average colour and its corresponding web colour (hexadecimal colour code) per pigments were identified and summarized in Table 1. Average colour in HSV, L*a*b* and Munsell colour space were also calculated and listed in Table 1.
The colour of pigments in L*a*b* colour space is typically measured using commercially available colourimeters. However, it only gives averaged L*a*b* information in the measured areas (typically 3-10 mm in diameter), as if a single point is measured. The partial resolution of colour measurement is limited to the size of sample area. The colour measurement is only reliable when the colour is uniform over the entire measurement area. If there is colour variation within the measuring area, only averaged colour information is available, which is often different from the real colour. Furthermore, colour information from an area smaller than the spatial resolution (i.e., the size of the measurement area) cannot be accessed.
To enhance the spatial resolution of colour identification, pixel-by-pixel colour analysis from optical microphotographs was attempted using image analysis software (PicMan). RGB, HSV, L*a*b*, Munsell colour and web colour information from any pixel of interest can be assessed and statistical colour analysis of ROI can be done. Even the colour information of a fine hair on paintings can be assessed. The theoretical spatial resolution is either determined by the diffraction limit of photography or the magnification of the photograph. Instead of a single set of numbers corresponding to the average colour from the colourimeters, colour distribution and the degree of colour inhomogeneity within ROIs can be assessed.
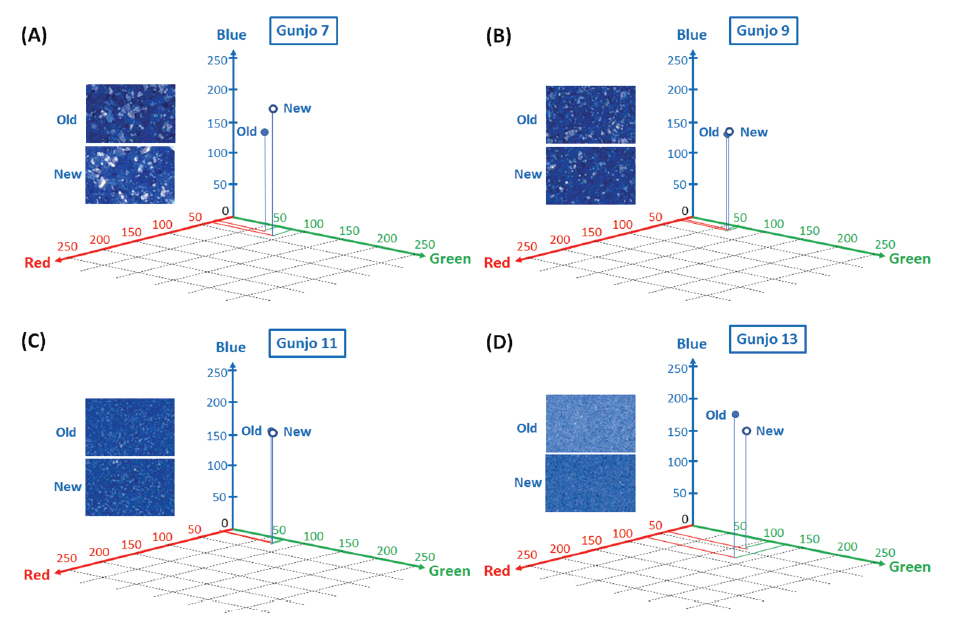
Figure 7(A-D) shows the average colour difference between old and new Gunjo pigments with four different grain sizes and their corresponding points in 3D RGB coordinates. RGB axes range from 0 to 255 (28 = 256 level). An RGB average of 152,100 pixels per image in 3.0 mm x 3.0 mm ROIs is obtained from the image analysis of optical photomicrographs. Gunjo 7 (coarse) and Gunjo 13 (extremely fine) pigments showed significant colour differences compared to the other Gunjo pigments with medium and fine grains (Gunjo 9 and Gunjo 11). Average colour cannot properly represent the colour variation within ROIs.
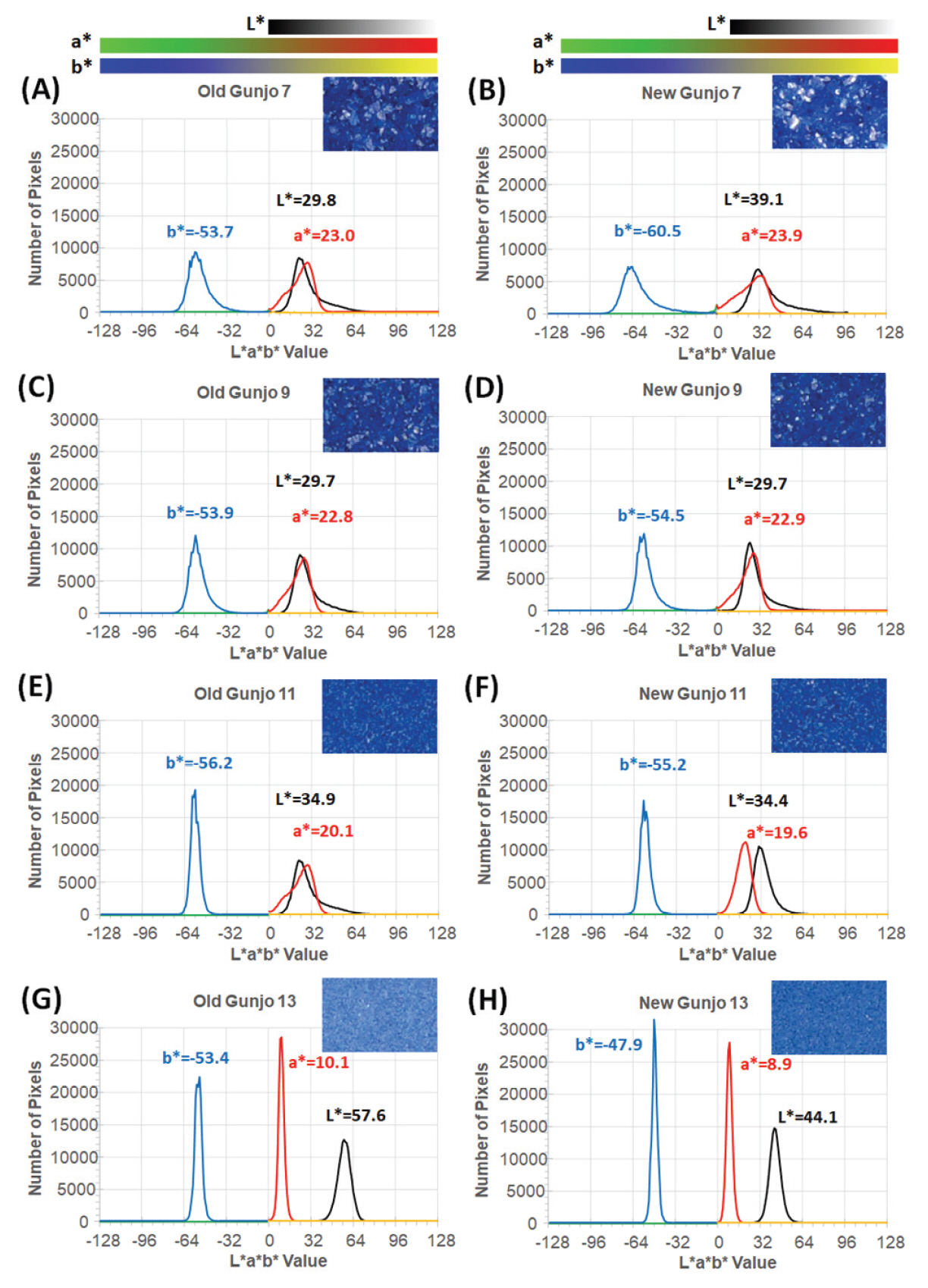
Figure 8(A-H) shows L*a*b* histograms (of 152,100 pixels per image) demonstrating colour distribution of pigments in 3.0 mm x 3.0 mm ROIs obtained from image analysis of optical photomicrographs. As seen from the histograms, the colour distribution of each pigment in L*a*b* colour space can be identified and compared. Uniform coloured pigments show very narrow distribution on each L*, a* and b* axis as seen in old Gunjo-13 (G) and new Gunjo-13 (H). Colour distribution within a measurement area can be analysed. The measurement area can be extended to the entire image. Statistical analysis and histogram generation in RGB, HSV, Munsell colour, web colours can be done in a similar way.
The colours of Gunjo 7 with coarse grains and Gunjo 13 with extremely fine grains from the old and new sets showed significant colour differences. For Gunjo 7, the notable colour difference could be due to the heterogeneity of the coarse pigment. For Gunjo 13, the notable colour difference is likely due to the crystallite growth that are particularly densely crowded in the small particle sizes.
Crystallites in old pigments were found particularly crowded in the fine particles of Gunjo 11 and 13. Comparing the colour data of old and new Gunjo 11 and 13, we notice an increase in b* (bluer), increase in a* (greener) and an increase in L* (lighter). This result slightly differs from our initial hypothesis, where the discolouration of old pigments was observed to be a paler blue. In contrast, comparing the old and new Gunjo 7 and 9, the colour change is the opposite: the b* value decreases (less blue), while L* decreases or shows no change (darker) and a* decreases or shows no change (redder). Although crystallites are also found at microscopic scale in Gunjo 7 and 9, they are sparsely distributed and do not seem to have much effect on the final colour. The accuracy of the colour change for Gunjo 7 and 9 appears to be limited by the heterogeneity of the coarse particles, hence their readings shall at best be interpreted as an average value.
Since we do not have photographs of the pigments at the time of purchase, it is difficult to prove that the same pigment changed colour with time, or the product colour varies between manufacturing batches over the years. By capturing photos of the pigments and performing an image analysis on a yearly basis, it will be possible to map out the rate of colour change for these pigments as a future research endeavour.
Our current work reports an interesting discolouration of blue Japanese glass pigments synthetically made for artistic use like nihonga or Japanese paintings. Two sets of blue pigments from the same manufacturer in Japan, purchased at two different times at least 8 years apart, showed a colour difference. The old set appears to be paler in colour than the new set. These pigments, though labelled under the marketed name Gunjo (群青), are not chemically ultramarine or azurite. Their chemical composition is characterized by SEM-EDS and Raman spectroscopy as cobalt aluminate spinels in leaded glaze. The pigments are manufactured by melting metal oxides into leaded glass at high temperatures, then grinding and pulverizing the product into different particle sizes, producing different shades of blues. Such lead-glazed spinel pigments seem to be characteristic of pigments commercially synthesized in Japan, probably already available since the late Shōwa period. It is unusual for discolouration to occur on such highly durable and chemically resistant pigments; hence an investigation was launched to study this phenomenon.
SEM reveals distinct crystallites (about 1-2 microns) in the old pigments, not seen in the new pigments. These crystallites when densely crowded on fine pigment particles may also be visible with confocal light scanning microscopy. The crystallites, characterized as lead hydroxycarbonate or similar, were deduced as new growths rather than part of the pigment formulation. Although Raman spectroscopy also detects lead hydroxycarbonate in the new pigments, the amount detected is very low and they do not appear as distinctive crystals when observed under the SEM. Pigment stability can be affected by various environmental factors such as heat, light, humidity, salinity, and CO2 concentration in the atmosphere, or by the manufacturing process and pigment formulation. In this case, our findings suggest that the crystallite growth in the old pigments is more likely due to the storage environment of the pigments. The release of acidic vapour from the wooden cabinet where the pigments were stored and the fact that the pigments were left largely undisturbed for a long period of time in this environment provide the conditions for crystallite growth.
From the end user’s point of view, the colour consistency of pigments is important for conservation, restoration or artistic work. Colour change is subjective to the human eye and a quantitative method is required to document colour changes objectively. The colours of the old and new blue pigments were measured by using an image analysis software (PicMan) to analyse photomicrographs of the pigments. Compared to a single chromaticity point data, this colour analysis technique statistically analyses over a larger area, taking into account colour variations within ROIs of any size (single pixel to entire image). From the graphed results, we can clearly observe the grain size dependence on colour change, where the smaller grain size shows lighter colour. Blue pigments with coarse and extremely fine grains showed noticeable colour change compared to pigments with medium and fine grain sizes. It was found that the crystal growth did not have much effect on the colour change in coarse pigments Gunjo 7 and 9. With regards to the finer pigments Gunjo 11 and 13 where crystallites are highly concentrated, the colour of the old pigment becomes lighter and bluer.
ACKNOWLEDGEMENTS
The authors are grateful to Bodil Unckel, HCC Objects Conservator, for sourcing the new pigment set, and Claire Lim, Paintings Conservator from The Conservation Studio (Singapore) for providence of the old pigment set to the HCC. We would also like to thank Claire L. Hoevel, Senior Conservator of Paper from the Indianapolis Museum of Art, Newfields, for providing provenance information and physical samples of blue Japanese pigments that helped further our understanding of such spinel-based glazed pigments (note: data is unpublished and beyond the scope of this study) and for reviewing our paper. Lastly, the current study arises as a spin-off from a larger project on “Blue Pigment Reference Analytical Database”, a collaboration between the Heritage Conservation Centre (HCC) and Shaanxi Institute for the Preservation of Cultural Heritage (SIPCH) in 2017-2021, spearheaded by Zhou Ping, Head of Conservation, SIPCH.
Figure 2.
SEM images of old and new pigments with four different grain sizes, purchased before 2010 (old) and in 2018 (new), under various magnifications from 3000 x to 13000 x to show the surface topography of the crystallites.
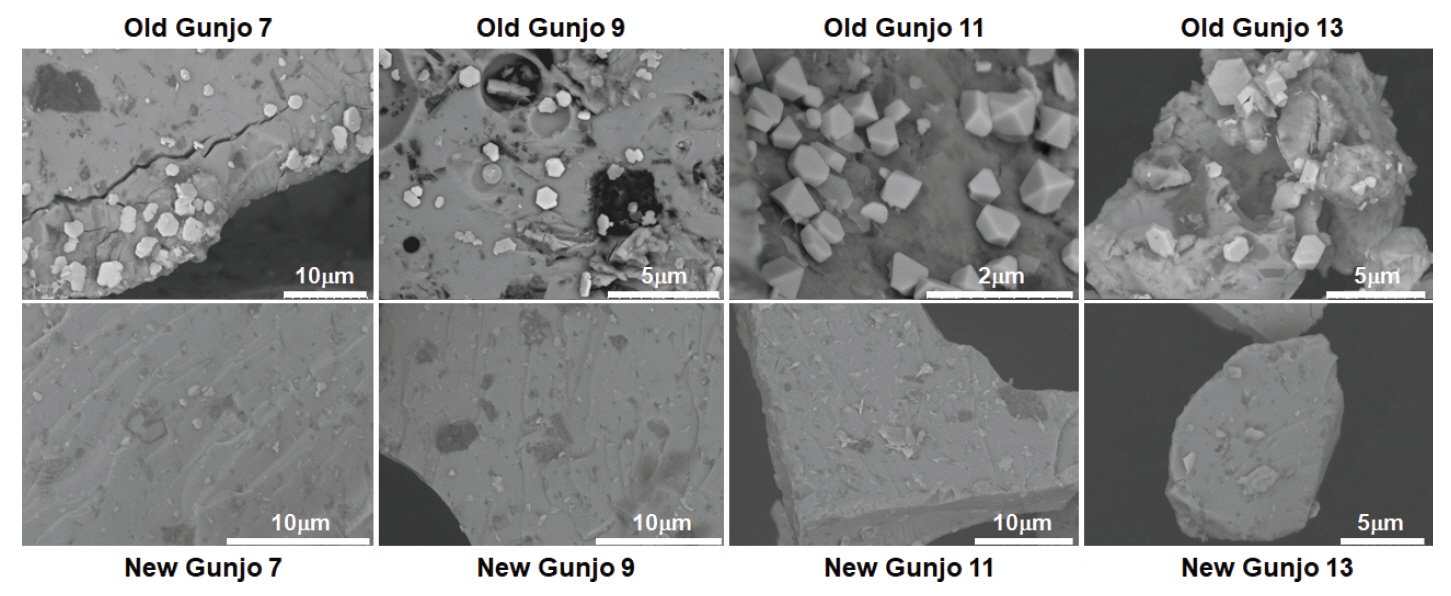
Figure 3.
SEM-EDS mapping of old Gunjo 13 pigment showing lead (Pb)-based crystallites on the surface of a Pb/Si glassy matrix. Dark spots correspond to cobalt (Co) and aluminium (Al), along with traces of zinc (Zn), chromium (Cr).
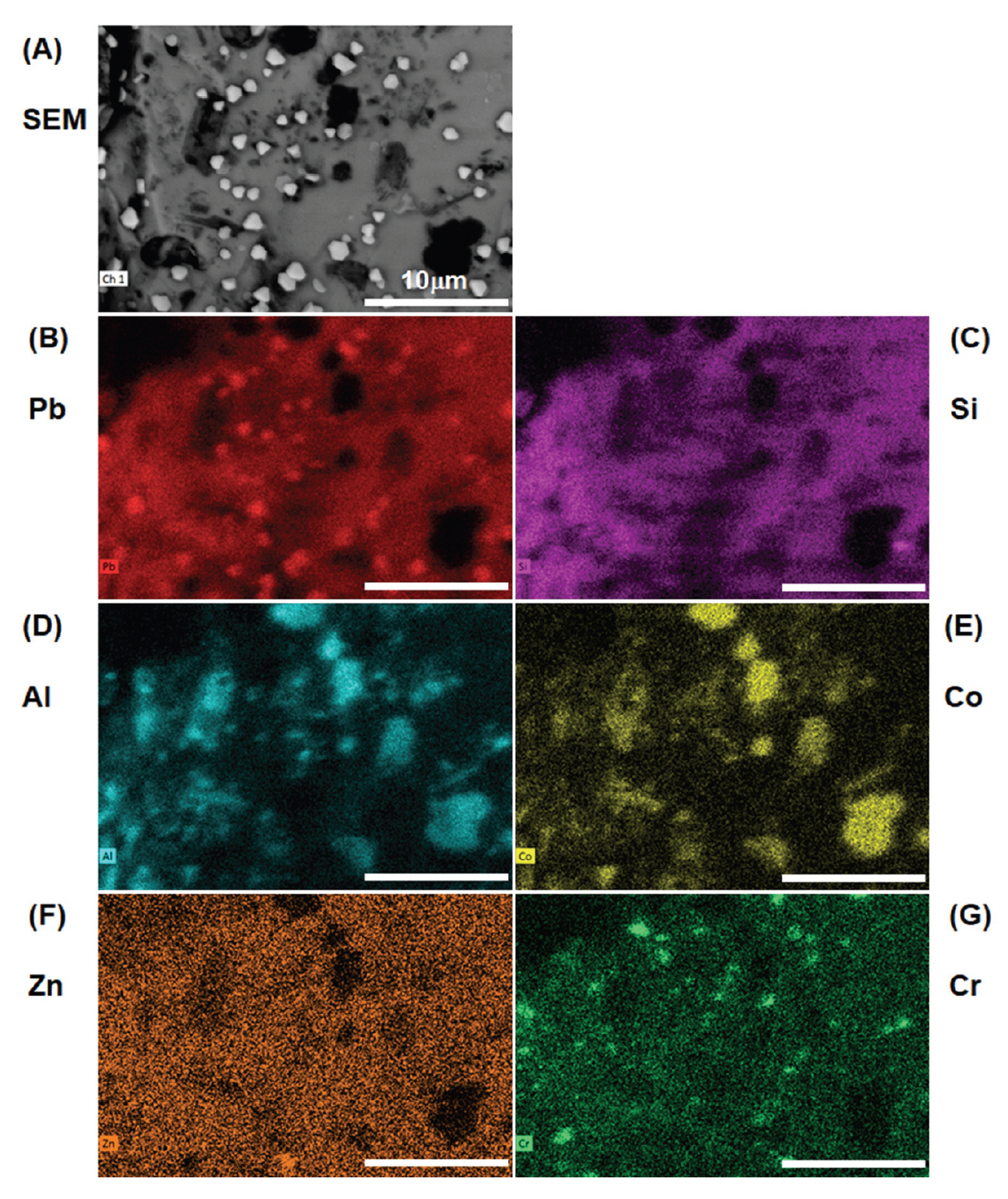
Figure 4.
Typical Raman spectrum of a (A) ‘blue spot’ (glassy matrix) and (B) ‘white spot’ (crystallite) in old and new pigment (Gunjo 13) acquired with a 532 nm laser.
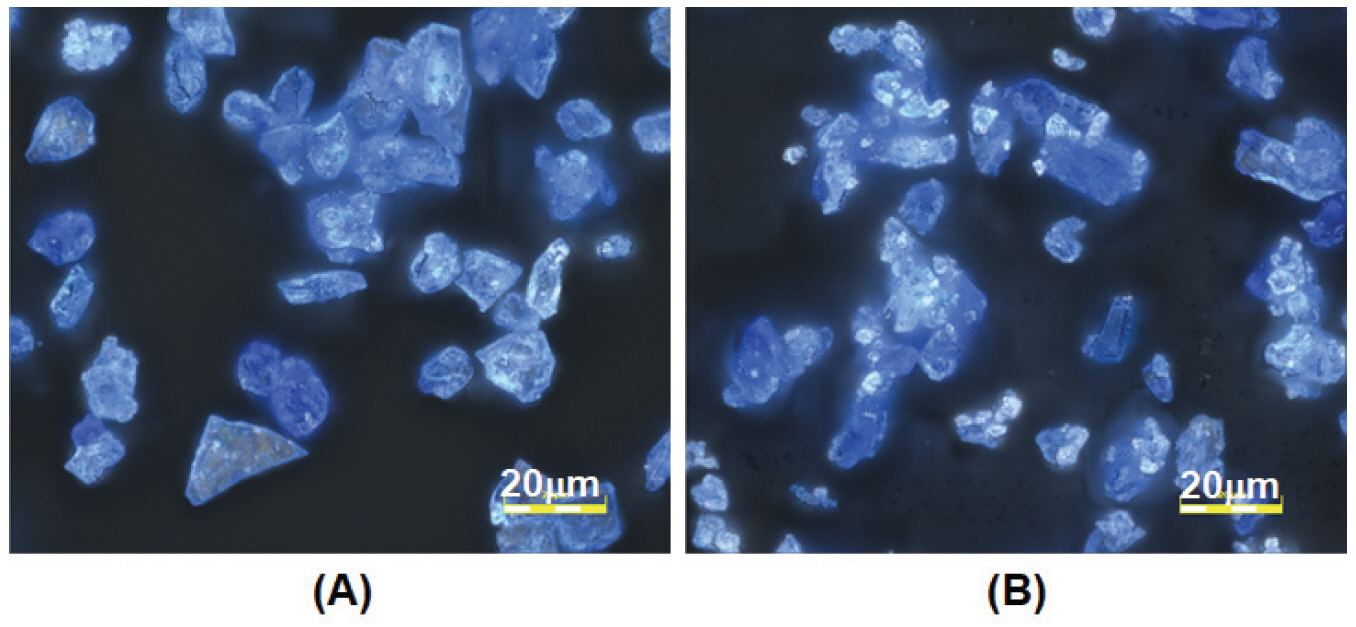
Figure 5.
Typical Raman spectrum of a (A) ‘blue spot’ (glassy matrix) and (B) ‘white spot’ (crystallite) in old and new pigment (Gunjo 13) acquired with a 532 nm laser.
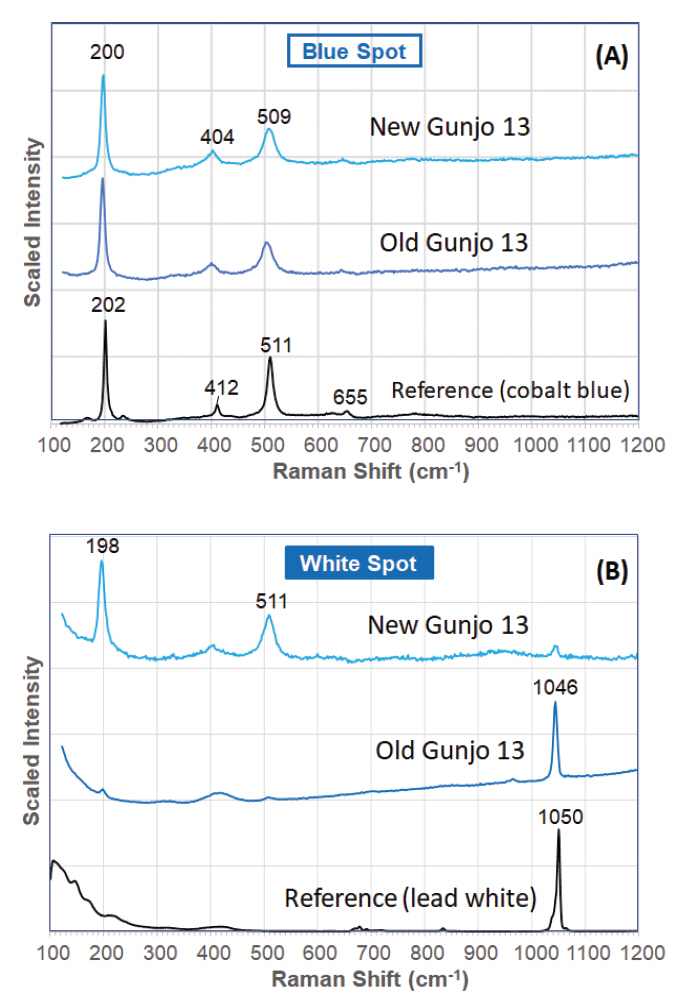
Figure 6.
Optical photomicrographs of the Gunjo pigments with four different particle sizes, purchased in 2000 (old) and 2018 (new), under the same illumination condition; 30 x magnification, 3.0 mm x 3.0 mm (9.0 mm2) in area.

Figure 7.
Average colour value of pigments in 3.0 mm x 3.0 mm ROIs obtained from image analysis of optical photomicrographs; (A) Old Gunjo 7 and New Gunjo 7, (B) Old Gunjo 9 and New Gunjo 9, (C) Old Gunjo 11 and New Gunjo 11, (D) Old Gunjo 13 and New Gunjo 13.
REFERENCES
Angelin, E.M., Bacci, M., Bartolozzi, G., Cantisani, E. and Picollo, M., 2017, Contemporary artists’ spinel pigments: Non-invasive characterization by means of electronic spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 173, 510–515.


Black, L. and Allen, G.C., 1999, Nature of lead patination. British Corrosion Journal, 34(3), 192–197.

Bouchard, M. and Gambardella, A., 2010, Raman microscopy study of synthetic cobalt blue spinels used in the field of art. Journal of Raman Spectroscopy, 41, 1477–1485.

Chua, L., Yan, G., Unckel, B. and Ping, Z., 2018, Analytical and terminological contribution to a database of commercial western and asian blue pigments, In: Presented at “Collections Care: Staying Relevant in Changing Times. ASEAN and Beyond”, Singapore,; Oct 23-25.
Cianchetta, I., Colantoni, I., Talarico, F., d’Acapito, F., Trapananti, A., Maurizio, C., Fantacci, C. and Davoli, I., 2012, Discolouration of the smalt pigment: experimental studies and ab initio calculations. Journal of Analytical Atomic Spectrometry, 27, 1941.

Comstock, C., 2016, Complex inorganic coloured pigments: Comparison of options and relative properties when faced with elemental restrictions. Journal of Surface Coatings Australia, December 2016, 10–30.
D’Ippolito, V., Andreozzi, G.B., Hålenius, U., Skogby, H., Hametner, K. and Günther, D., 2015, Colour mechanisms in spinel: Cobalt and iron interplay for the blue colour. Physics and Chemistry of Minerals, 42, 431–439.

Gonçalves, P.M., Pires, J., Carvalho, A.P., Mendonça, M.H. and Cruz, A.J., 2010, Theory vs practice: Synthesis of white lead following ancient recipes. In: Luís Urbano Afonso, editors. The Materials of the Image, the University of Lisbon, Lisbon, 185–200.
Hoevel, C.L., 2021, Private communication.
Kim, E.A., Kim, D.S., Hyen, J.H. and Kim, G.H., 2019b, Study on material characteristic evaluation of Sangpyeongtongbo coins in Joseon Dynasty using non-destructive analysis. Science and Engineering of Cultural Heritage, 14(1), 23–30. (in Korean with English Abstract)

Kim, E.A., Lee, J.H. and Kim, G.H., 2021, A characteristic analysis of glass beads in Geumgwan Gaya, Korea (I). Journal of Conservation Science, 37(3), 232–244.

Kim, G., Kim, J.G., Kang, K. and Yoo, W.S., 2019a, Image-based quantitative analysis of foxing stains on old printed paper documents. Heritage, 2, 2665–2677.

Lee, M.Y. and Wi, K.C., 2021, A study on the colour of natural solvent for the red colour reproduction of safflower. Journal of Conservation Science, 37(1), 13–24.

Llusar, M., Forés, A., Badenes, J.A., Calbo, J. and Monrós, G., 2001, Colour analysis of some cobalt-based blue pigments. Journal of European Ceramic Society, 1121–1130.

Nakagawa Gofun Enogu, 2021a, Developing new Iwa Enogu. http://nakagawa-gofun.co.jp/english/begin/development.html (August 1, 2021)
Nakagawa Gofun Enogu, 2021b, Pigment. http://nakagawa-gofun.co.jp/english/begin/about.html (November 6, 2021)
Niklasson, A., Johansson, L.-G. and Svensson, J.-E., 2008, The influence of relative humidity and temperature on the acetic acid vapour-induced atmospheric corrosion of lead. Corrosion Science, 50(11), 3031–3037.

Pigment Lab, 2021, The types and features of 4500 colors in pigment Tokyo - vol. 3. https://pigment.tokyo/article/detail?id=53 (November 30, 2021)
Robinet, L., Spring, M., Pagès-Camagna, S., Vantelon, D. and Trcera, N., 2011, Investigation of the discolouration of smalt pigment in historic paintings by micro-X-ray absorption spectroscopy at the Co K-edge. Analytical Chemistry, 83, 5145–5152.


Shin Iwa Enogu, 2021, http://nakagawa-gofun.co.jp/product/Iwa_enogu/Sin_Iwaenogu/index.html (August 1, 2021)
Smith, G.D., Derbyshire, A. and Clark, R.J.H., 2002, In situ spectroscopic detection of lead sulphide on a blackened manuscript illumination by Raman microscopy. Studies in Conservation, 47, 250–256.

Tang, Q., Zhu, H., Chen, C., Wu, J. and Shih, W., 2017, Preparation and characterization of nanoscale cobalt blue pigment for ceramic inkjet printing by Sol-gel self-propagating combustion. Materials Research, 20(5), 1340–1344.

The Color of Art Pigment Database, 2021, Quick reference index. http://www.artiscreation.com/color_index_index.html (December 21, 2021)
Yoneda, M., Gotoh, K., Nakanishi, M., Fujii, T. and Nomura, T., 2016, Influence of aluminum source on the colour tone of cobalt blue pigment. Powder Technology, 323, 574–580.

Yoneda, M., Gotoh, K., Nakanishi, M., Fujii, T., Konishi, Y. and Nomura, T., 2018, Solid‐state synthesis and characterization of cobalt blue core‐shell pigment particles. Journal of American Ceramic Society, 102, 3468–3476.

Yoo, W.S., Kang, K., Kim, J.G. and Jung, Y.-H., 2019, Development of image analysis software for archaeological applications. Advancing Southeast Asian Archaeology, 402–411.
Yoo, W.S., Kim, J.G., Kang, K. and Yoo, Y., 2021, Extraction of colour information from digital images towards cultural heritage characterisation applications. SPAFA Journal, 5, doi: 10.26721/spafajournal.2021.v5.690

- TOOLS
-
METRICS

-
- 5 Crossref
- 5,171 View
- 101 Download
-
Related articles in
J. Conserv. Sci.