A Study on the Preparation and Stability of Natural Cleaning Agents for Excavated Gold Leafed Clothing Relics
Article information
Abstract
The aim of this study was to develop a natural cleaning agent for the conservation treatment of excavated gold leafed clothing artefacts and to test the stability of its application. To this end, a natural cleaning agent was formulated by mixing the extracts of Platycladus orientalis with natural cleaning aids prepared using coconut oil, rice kernel oil, and castor oil. The prepared natural cleaning agent was found to have better cleaning power and stability as a cleaning agent for clothing relics, compared to Saponin, Decyl glucoside, and Triton X-100, which have been conventionally used as cleaning agents for excavated clothing relics. A comparison of the four types of cleaning agents showed that the natural cleaning agent prepared with the extracts of Platycladus orientalis has significantly better cleaning power compared to three low and high concentration cleaning agents that have been used as cleaning agents for clothing relics. The findings show that the pH of the prepared natural cleaning agent was neutral, ranging from 6.61 to 6.93, indicating very weak acidity, the natural cleaning agent did not cause any pH related problems to the fabric during or after cleaning, and the prepared natural cleaning agent can be used as a stable natural agent for clothing relics because it has better cleaning power than the other conventional cleaning agents even at a relatively low concentration of 0.5% to 1.5%, a treatment time of 30 min., and a temperature below 30°C, a temperature which is not damaging to clothing relics.
1. INTRODUCTION
Gold leafed clothing artifacts, which are decorated with gold leaf on fabrics, are subject to biological damage due to the environment in which they were found, storage processes, airborne contaminants, and exposure to ultraviolet light after excavation. In particular, most gold leafed clothing relics excavated from tombs have been frequently biologically damaged by microorganisms caused by waterlogging or moisture in the soil due to the burial environment, and their corrosion and decay are accelerated due to contaminants that have been fixed by various causes. Cleaning step is essential for the conservation and management of clothing relics as most contaminants undergo a rapid process of oxidation after excavation and thereby cause the deformation and loss of their original colors and physical form, further damaging the relics overtime and reducing their physical performance.
Research on the wet cleaning of gold leafed clothing and excavated clothing relics from the 1970s to the early 2000s includes the anionic surfactant LAS (Sheila, 1992), anionic + nonionic surfactants (Kim, 1998), nonionic surfactant Triton X-100 (Yeongju, 1998), and Saponin (Park, et al., 2008; Kim, et al., 2010; Oh, 2010). Recently, the most commonly used wet cleaning method is to pre-soak clothing relics for 30 min. in a cleaning solution made by mixing Saponin (0.5 g/L), a natural non-ionic surfactant, and SCMC (Tylos C 300, 5.0 x 10−3 g/L) as an anti-re-adhesion agent, followed by cleaning with a sponge (Oh, et al., 2007). However, the cleaning effectiveness and stability of these methods have been still somewhat questionable. Although there have been studies on the cleaning of clothing artifacts using natural surfactants containing various substances (Jung, et al., 2010), there are still few studies on the natural cleaning of excavated clothing relics (Lee, et al., 2020; Baek, et al., 2017) with some specific results. The natural cleaning agents that have been studied so far are formulated using vegetable oils, essential oils, herbs, and natural plant powders without artificial additives such as preservatives or synthetic surfactants. Despite these advantages, there still remain some questions about whether these natural cleaning agents are safe to use on excavated organic relics (Kang, et al., 1994) or whether they can stably remove contaminants that may be problematic in the future.
The basic preparations for utilizing Platycladus orientalis as a natural surfactant cleaning agent have already been done since studies have been conducted on its main constituents, sabinic acid, uniferic acid, hinokitiol, quercetin, saponin (Kim, et al., 1998) and thujene, pinene and other flavonoids with excellent antioxidant activities (Ahn, et al., 1998), and hinokitiol and essential oil (Cavaleiro, et al., 2001) components of conifers with insecticidal, flavoring, pharmaceutical, aromatic (Lee, 1999) and bactericidal activities (Gil, 1993).
Based on these contents, the purpose of this study was to prepare a natural cleaning agent by mixing extracts of Platycladus orientalis and cleaning aids with different active ingredients and to test its cleaning power and stability on relics. The various formulations were prepared and compared with three other conventional cleaning agents, focusing on their cleaning power and stability of the relics when applied to conservation treatments of the relics.
2. THE MATERIALS AND THE METHOD
2.1. Reagents and Instruments
The solvents used in this study were methyl alcohol (99.9%), ethyl alcohol (99.9%), potassium hydroxide, citric acid, H2O2, and NaOCl from Samchun Chemicals Co., Ltd. and Saponin, Triton X-100, and Decyl glucoside from Basic Science Co., Ltd. For all the experiments, analytical grade chemical and doubly distilled, demineralized water were used for dilution and solution preparation.
The surface condition before and after the cleaning experiments was checked using a high resolution digital camera (Canon G10, JPN), and the removal of contaminants on the surface of the fabrics and the peeling off of the gold leaf were checked using a portable digital microscope (Portable Digital Microscope DG-3X, Scxlar, JPN). The pH of the cleaning agents was measured using a Testo (Testo 206, DEU), and the color changes before and after cleaning were determined using a spectrometer (Konica-Minolta CM-2600d, JPN) using a D65 light source with a UV component to remove specular reflected light, with an observation field of 10° and a measuring diameter of 8 mm. Glossiness was measured using a gloss meter (GLPSS METER, IG-320, HORIBA, JPN) in accordance with the provisions of KSL 2405, with a scanning light source LED (wavelength: 880 nm), with a scanning angle of 60°, a receiving angle of 60°, and a cross sectional area of 12 X 6 mm, to the nearest 0.1 unit.
2.2. Materials
2.2.1. Specimens
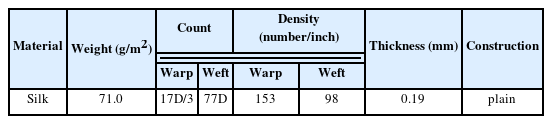
The test fabrics were purchased from the Korea Apparel Testing and Research Institute (KATRI) and were cut into a size of 10.0 X 10.0 cm (Table 1).
The gold leaf specimens were prepared with three layers of gold leaf, glue, and medium to create the same structure as the excavated relics. It was manufactured by bonding traditional Korean paper and gold leaf using animal hide glue. First, a dilute animal hide glue solution of pH 5.30 (2.0 g of animal hide glue, 100.0 mL of distilled water, 1.0 g of alum) was applied to the traditional korean paper, and 15% glue was applied to the surface of the gold leaf, followed by adhesion of the traditional korean paper and gold leaf. On top of this, a 1 μm thick gold leaf was fixedly glued using a brush and dried. And the surface of the gold leaf was polished to smooth the surface, and then the gold leaf was cut into 5.0 x 5.0 cm and used as gold leaf specimens.
2.2.2. Artificial Contaminants
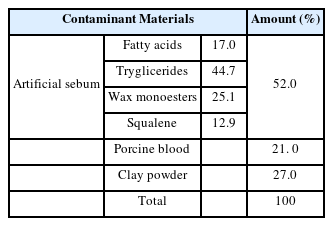
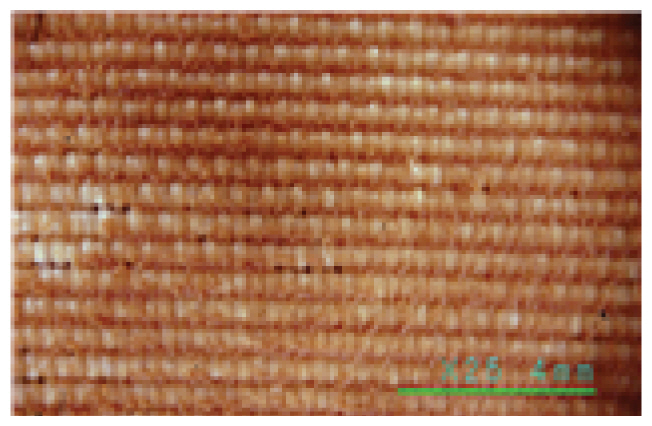
Since the contaminants on the excavated clothing are found to be decomposed resinous hydrolysates of animal fats and proteins containing fats, proteins, and inorganic salts such as Mg, Ca, S, and Fe resulting from corpse decomposition and microbial decomposition, artificial contaminants were prepared and used according to previous studies (Jo, et al., 2011; Lee, et al., 2000) and KSC IEC60456 (artificial mixed contaminants based on contaminants). The artificial sebum was composed of 17.0% of fatty acids, 44.7% of tryglicerides, 25.1% of wax monoesters, and 12.9% of squalene according to KSC IEC60456, which were mixed and stirred while raising the temperature to 80°C and cooling the homogenized liquid to room temperature before use. The artificial mixed contaminants were prepared by mixing 52.0% of the artificial sebum with 21.0% of porcine blood and 27.0% of clay powder to form a dilute clay. The blood was prepared by adding ammonium citrate to prevent the solidification of the porcine blood while matching it as closely as possible to the current state of the blood on the relics. The clay was collected from the reddish yellow clay of Gaya Mountain behind Hanseo University, mixed with sterile saline solution, passed through a standard sieve of 750 μm, and dried in a 36°C hot air desiccator for more than 48 hours before being repulverized (Table 2).
The artificial mixed contaminants prepared in this way were applied to the silk specimens and gold leaf specimens at 2.0 g each, which were completely fixed in a desiccator at 60°C for more than 48 hours to be used as contaminant specimens.
2.3. Preparation of the Extracts of Platycladus orientalis
The washed leaves of Platycladus orientalis were dried and ground to a fine powder. The ground powder was separated to 50 mesh or less, which was extracted three times with 10 times distilled water at 80°C for 3 hours with stirring. Then, the extracts were extracted with 70% methanol and 70% ethanol solvents using the same method as the aqueous extraction, and the extracts were pooled and filtered through filter paper (Whatman No. 2). The filtered extracts were concentrated under reduced pressure at 45°C with a rotary vacuum evaporator to remove all the solvents used, and the concentrated solution was freeze dried (yield 55.0%).
2.4. Preparation of Cleaning Agents from the Extracted Mixture
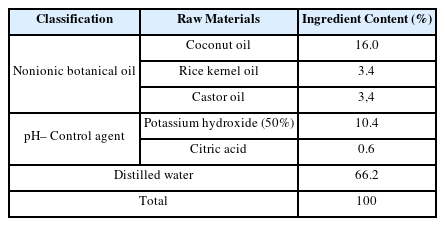
The cleaning aid to be mixed with the extracts of Platycladus orientalis was prepared according to the mixing ratio in Table 3. 16.0 g of coconut oil, 3.4 g of rice kernel oil, and 3.4 g of castor oil were heated to 75°C with stirring. 10.4 g of 50% KOH solution was added to this and stirred for 30 min. at 70°C. When the solution gelled past the trace state, the heat source was removed and 66.2 g of distilled water was added while maintained at 70°C in an incubator for 1 hour. In the secondary gelation state, the pH of the cleaning aid was adjusted to 7.0 to 7.5 using citric acid and stabilized at room temperature for 72 hours. Natural cleaning agents for excavated clothing relics were prepared by mixing the prepared cleaning aid with a mixture of 20 g of powdered Platycladus orientalis extracts and 1 L of distilled water which was bleached for 24 hours by adding 4.0 g of H2O2 and 4.0 g of NaOCl.
2.5. Conditions of Use of Conventional Cleaning Agents
Three cleaning agents - Saponin, Decyl glucoside, and Triton X-100, which have been conventionally used to date for the cleaning of excavated clothing relics - were used as controls for the natural cleaning agents extracted from Platycladus orientalis. The concentrations of the prepared cleaning agents were 0.05%, 0.5%, 1.0%, and 1.5%, and the average cleaning temperatures were 20°C, 25°C. 30°C, and 35°C, and the cleaning time was 30 min.
3. RESULTS AND DISCUSSION
Main factors in selecting a cleaning agent include the temperature and time of cleaning, the critical micelle concentration (CMC, the surfactant concentration at which micelles begin to form), the degree of secondary contamination after cleaning, the amount of cleaning agent remaining after cleaning, and the pH at the time of cleaning. In particular, the cleaning time and pH of a cleaning agent are very important factors that determine whether the relics can be stably preserved after cleaning, so it is essential to test and adjust them first. A highly alkaline cleaning agent is very effective for removing contaminants, but it can also cause problems with the stability of fibers such as excavated clothing during and after cleaning, which is why it is important to keep a cleaning agent close to neutral. In other words, it is essential to focus on the near neutralization of a cleaning agent for excavated clothing relics, and to add or adjust other factors to complete the cleaning agent when considering the stability of the relics.
3.1. Results on the Cleaning Power and Stability of Conventional Cleaning Agents
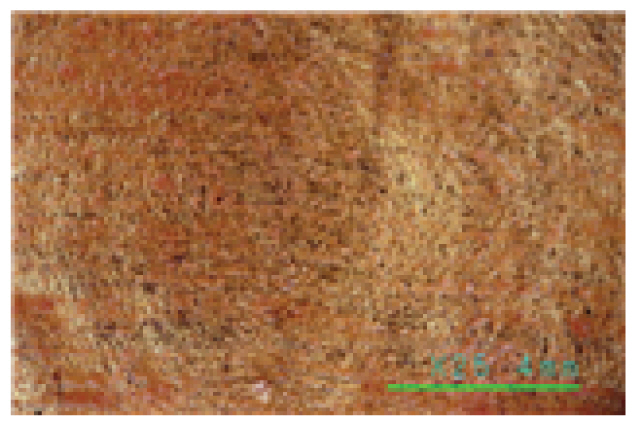
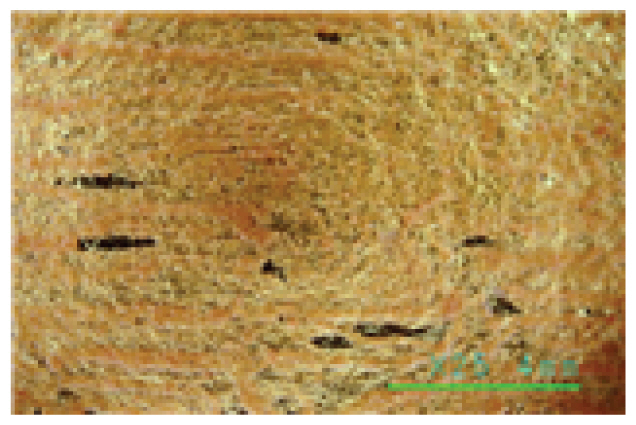
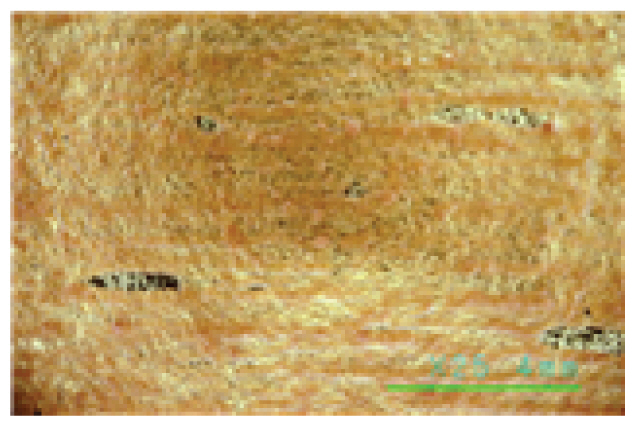
Saponin, Decyl glucoside, and Triton X-100 are typical cleaning materials used in previous studies and have been formulated and used for cleaning at concentrations as low as 0.05%. The pH of cleaning agents at a concentration of 0.05% was 6.25~6.35 for Saponin, 7.06~7.13 for Decyl glucoside, and 6.80~6.90 for Triton X-100, indicating relatively stable weak acidity and weak alkalinity. The reason why they are used at a low concentration of 0.05% would be for the stability of the fiber relics, and all the three cleaning agents were neutral, weakly acidic, and weakly alkaline, so they are not expected to have a physical impact on the fiber organics. As can be seen in Table 4, a microscopic examination of the cleaning power shows that these cleaning agents have little cleaning effect. These cleaning agents removed very few contaminants on both the fiber and gold leaf specimens, with some peeling off of foreign substances from the surface of the specimens due to shaking of the solution, even after 30 min. of cleaning at 35°C, a relatively high temperature for artifact cleaning. As shown in the photographs of the gold leaf in Table 4, it appears difficult to remove the contaminants on the gold leaf specimens using conventional cleaning agents. The fact that most of the artificial contaminants with low adhesion and wetness to the metal remained even after cleaning indicates that the three cleaning materials - Saponin, Decyl glucoside, and Triton X-100 - acted more like distilled water with normal solubility than they did as cleaning agents. The findings show that a low concentration of 0.05% that has been used so far can be used to reliably rinse away some loose contaminants rather than clean them.

Cleaning power of Saponin, Decyl glucoside, and Triton X-100 by concentration and temperature (0.05%, 35°C, 30 mins)
The chromaticity measurements showed that Triton X-100, which had the largest change in L* and b* values before and after cleaning of silk fabrics, only showed a difference of less than 8.0 and a change in ΔE*ab value of less than 9.0, indicating that there was little change in chromaticity due to the removal of contaminants. For the gold leaf specimens, the changes in L* and b* values before and after cleaning with Decyl glucoside and Triton X-100 were up to 17.28 and −23.32, and the ΔE*ab value was 31.43, but this cannot be considered as changes in chromaticity due to cleaning because the changes were for specimens with the peeling off of the gold leaf.
When it comes to glossiness, the measured glossiness after the contaminants were applied to the gold leaf specimens and cleaned with the conventional cleaning agents - Saponin, Decyl glucoside, and Triton X-100 - increased overall, with a relatively large difference of 14.3 for Saponin, but this change was also found to be a change in glossiness due to the occurrence of the peeling off on the surface of the gold leaf layers due to temperature changes.
3.2. Measurements of Cleaning Power and Stability with Platycladus orientalis Extract Cleaning Agents and Conventional Cleaning Agents
3.2.1. Cleaning Power and Stability on Silk Fabrics
Natural cleaning agents were prepared with extracts of Platycladus orientalis as the main ingredient for cleaning, and no conventional surfactants were used to enhance their naturalness; instead, only coconut, rice kernel, and castor oil were used as cleaning aids. The natural Platycladus orientalis extract cleaning agents and conventional cleaning agents were prepared at concentrations of 0.5%, 1.0%, and 1.5% to check their cleaning performance, the stability of the cleaning agents themselves, and the stability of the fabrics after cleaning. The average pH of the prepared cleaning agents was found to be neutral at 6.56~7.21 for Platycladus orientalis cleaning agents, slightly acidic at 5.53~6.42 for Saponin, slightly alkaline at 7.26~8.07 for Decyl glucoside, and slightly alkaline at 7.05~8.06 for Triton X-100. All the four cleaning were found to be neutral, weakly acidic, and weakly alkaline, indicating that they would be unlikely to have a significant effect on the target fiber organics.
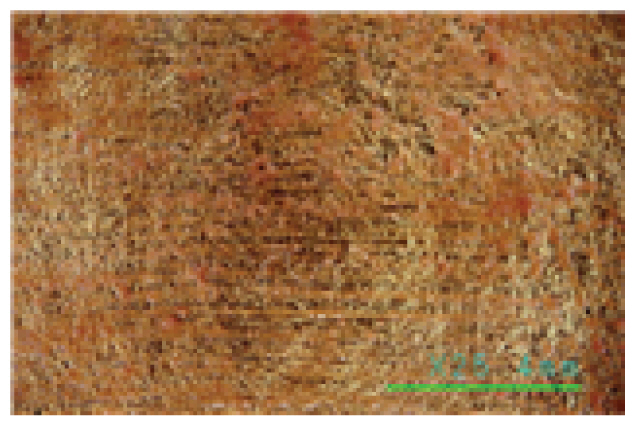
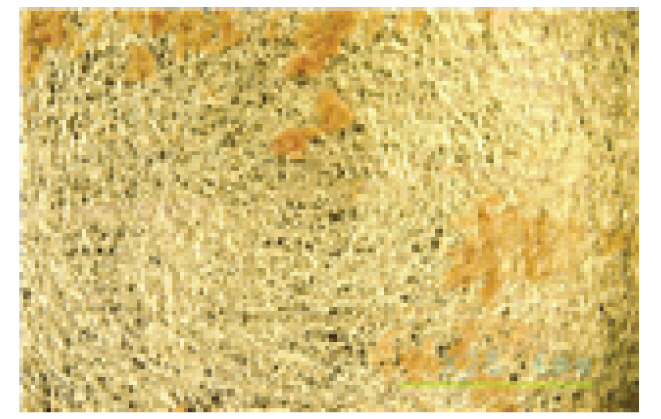
The microscopic examination and colorimetric measurements of the prepared Platycladus orientalis cleaning agent showed that the agent removed the artificial contaminants applied to the fabric even at a concentration of 0.5% and a cleaning temperature of 20°C, as shown in Table 5, while the high concentration adjusted conventional cleaning agents of Saponin, Decyl glucoside, and Triton X-100 failed to remove the contaminants even at a higher concentration of 1.5% and a cleaning temperature of 35°C as shown in Table 6. The Platycladus orientalis cleaning agent, unlike the other agents, showed visible color change with the highest ΔE*ab value of 45.96, indicating its excellent cleaning performance.
Cleaning power of the Platycladus orientalis extract cleaning agent by concentration and temperature (20°C, 30 mins)
3.2.2. Cleaning Power and Stability on Gold Leaf
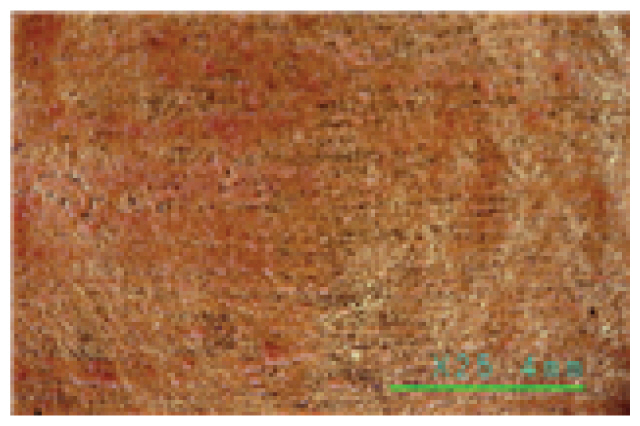
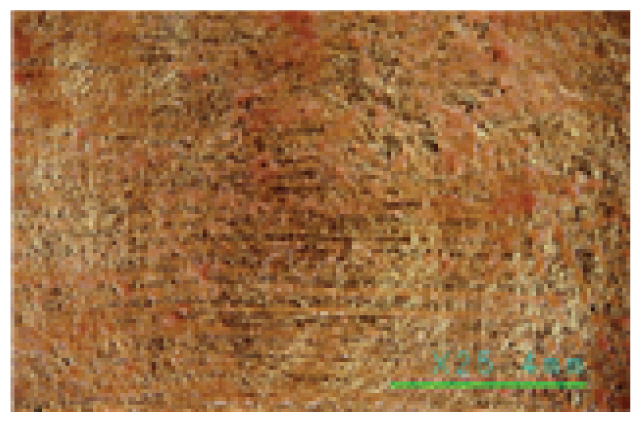
The microscopic examination of the gold leaf specimens showed some peeling off on all the gold leaf specimens within 30 min. of cleaning not only in the Saponin, Decyl glucoside, and Triton X-100 cleaning agents, but also in the Platycladus orientalis cleaning agents at a cleaning temperature above 30°C.
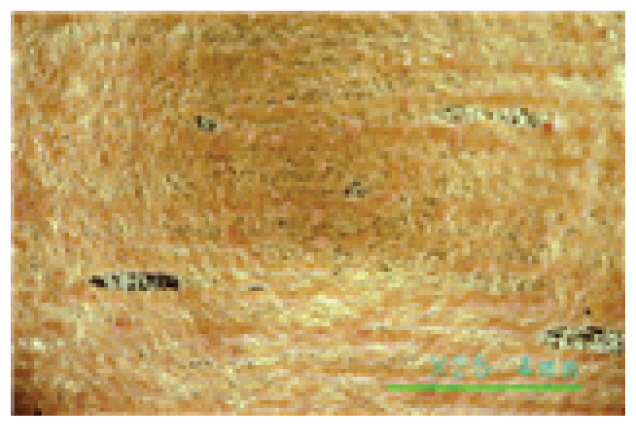
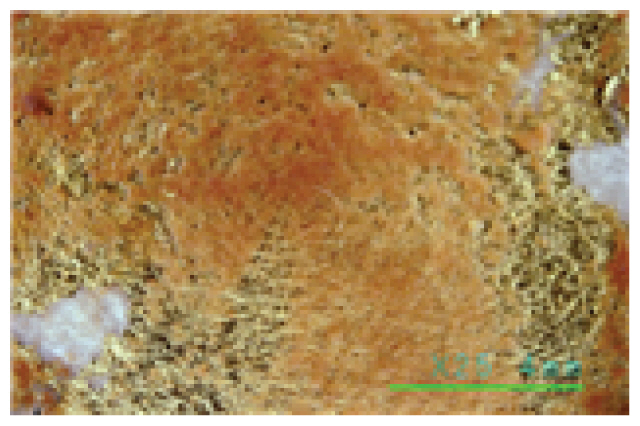
In particular, the Saponin and Decyl glucoside cleaning agents seemed to have some cleaning power above 25°C at high concentrations of 1.0% or more, but at this temperature, even at a low concentration of 0.5%, some of the gold leaf began to peel off. The Triton X-100 cleaning agent was found to be unsuitable for specimens with gold leaf, as some of the gold leaf began to peel off at 20°C, even at a low concentration of 0.5%, as shown in Table 7. On the other hand, the Platycladus orientalis cleaning agent showed excellent cleaning power at all concentrations of 0.5%, 1.0%, and 1.5%, and at a cleaning temperature as low as 20°C, and no peeling off of the gold leaf was found below 30°C, as shown in Table 8. In particular, the Platycladus orientalis cleaning agent showed a stable cleaning effect even at a concentration of 1.0%, a cleaning temperature of 20°C, and a cleaning time of 30 min..

Cleaning power of the Saponin, Decyl glucoside, and Triton X-100 cleaning agents by concentration and temperature (0.5%. 25°C, 30 min.)

Cleaning power of the Platycladus orientalis extract cleaning agents by concentration and temperature (1.0%, 20 to 35°C, 30 min.)
ΔL* and Δb* values before and after cleaning with the Platycladus orientalis extract and natural cleaning aids were up to 40.0 or more at 20°C and 25°C, especially the cleaning agents with the 1.0% Platycladus orientalis extract at 20°C showing 41.85 of ΔE*ab on silk fabrics and 37.34 of ΔE*ab on gold leaf specimens. At these temperatures and concentrations, the cleaning agents with the extracts of Platycladus orientalis and natural cleaning aids showed a significant increase in brightness, with ΔL* values of 29.55 and 24.36. In particular, as shown in Table 9, changes in Δa* values closely related to red pollutants were shown to be -22.59 and -20.46, indicating excellent results in removing artificial pollutants (Δb* 19.68 and 19.17).
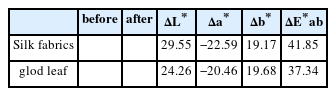
Chromaticity before and after cleaning silk fabrics and gold leaf using Platycladus orientalis extract cleaning agents (1.0%, 20°C, 30 min.)
The glossiness measurements showed that the changed glossiness after cleaning the gold leaf specimens with contaminants applied increased in the order of Platycladus orientalis extract cleaning agents, Triton X-100, Decyl glucoside, and Saponin. Specifically, 1.0% of Saponin cleaning agent showed a change in glossiness of 3.9 (3.7→ 7.6) at 25°C, 1.5% of Decyl glucoside showed a change in glossiness of 5.5 (3.7→9.2) at 25°C, and 1.5% of Triton X-100 showed a change in glossiness of 6.8 (3.6→10.4) at 25°C. The 1.0% of Platycladus orientalis extract cleaning agent had a change in glossiness of 12.5 (3.6→16.1) at 25°C, indicating that the specimens cleaned with the Platycladus orientalis extract cleaning agent had the highest glossiness.
4. CONCLUSION
The purpose of this study was to determine the applicability of using a cleaning agent prepared with Platycladus orientalis extracts and natural cleaning aids to preserve gold leafed clothing relics. As for cleaning power, the previously studied and practiced cleaning agents - Saponin, Decyl glucoside, and Triton X-100 at a concentration of 0.05% - failed to remove all artificial contaminants on the specimens as measured by the results of microscopy and colorimetry after cleaning. These cleaning agents were also unsuccessful on the gold leaf specimens, as they caused the gold leaf to peel off during the decontamination step, making them unsuitable as a cleaning agent. Regarding chromaticity, there was little change in the color of the silk fabrics, and as for glossiness, there was a temperature dependent peeling off of the gold leaf layer.
The Platycladus orientalis cleaning agent and Saponin, Decyl glucoside, and Triton X-100 cleaning agents using the previously studied cleaning materials at the same high concentrations as the Platycladus orientalis cleaning agent were expected to exhibit no pH induced problems, but even at high concentrations, Saponin, Decyl glucoside, and Triton X-100 removed very few contaminants, whereas the prepared Platycladus orientalis cleaning agent removed artificial contaminants adhering to the surface of the fabric specimens, even at relatively low temperatures.
The stability measurements showed that all the four types of cleaning agents caused the gold leaf to peel off within 30 min. of the cleaning time when the cleaning temperature exceeded 30°C. For cleaning below 30°C, cleaning the specimens with the Platycladus orientalis cleaning agent most closely approximated the chromaticity and glossiness of the gold leaf specimens before the contaminants were applied, suggesting that this cleaning agent can clean gold leafed clothing relics very stably. Overall, the Platycladus orientalis cleaning agent was found to exhibit a superior cleaning performance compared to the three other previously studied high concentration cleaning agents. Considering its usefulness in the cleaning of metal specimens, the Platycladus orientalis cleaning agent was also found to be effective in cleaning clothing relics containing both silk fabrics and metal threads.